CLARIFOAM EF
-
sulfur and
sulfacetamide sodium aerosol, foam
Onset Therapeutics, LLC
----------
Clarifoam EF
FOR EXTERNAL USE ONLY
Rx Only
DESCRIPTION
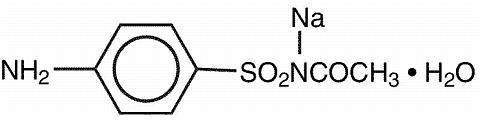
Sodium sulfacetamide is a sulfonamide with antibacterial activity while sulfur acts as a keratolytic agent. Chemically, sodium sulfacetamide is N-[(4-aminophenyl) sulfonyl]- acetamide, monosodium salt, monohydrate.
The structural formula is:
Each gram of CLARIFOAM® EF (sodium sulfacetamide 10% and sulfur 5%) Emollient Foam contains 100 mg of sodium sulfacetamide and 50 mg of sulfur in an aqueous based emollient foam vehicle containing cetyl alcohol NF, emulsifying wax NF, lactic acid USP, methylparaben NF, propylene glycol USP, propylparaben NF, steareth-10, water USP. Also contains: Propellant HFA-134A (1,1,1,2-tetrafluoroethane).
CLINICAL PHARMACOLOGY
Sodium sulfacetamide exhibits antibacterial activity. The most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory which is based on the fact that sulfonamides act as competitive antagonists to para-aminobenzoic acid (PABA), an essential component for bacterial growth. While absorption through intact skin has not been determined, sodium sulfacetamide is readily absorbed from the gastrointestinal tract when taken orally and excreted in the urine, largely unchanged. The biological half-life has variously been reported as 7 to 12.8 hours.
It is estimated that 1% of topically applied sulfur is absorbed. Although the exact mode of keratolytic activity of sulfur is unknown, it is reported to result from the interaction of sulfur with the cysteine content of keratinocytes. In combination with sulfacetamide, sulfur has been reported to inhibit the growth of Propionibacterium acnes thereby reducing the associated inflammation.
INDICATIONS
CLARIFOAM EF Emollient Foam is indicated in the topical control of acne vulgaris,acne rosacea and seborrheic dermatitis.
CONTRAINDICATIONS
CLARIFOAM EF Emollient Foam is contraindicated for use by patients having known hypersensitivity to sulfonamides, sulfur or any other component of this preparation. CLARIFOAM EF Emollient Foam is not to be used by patients with kidney disease.
WARNINGS
Although rare, hypersensitivity reactions to products containing sodium sulfacetamide may occur, including Stevens-Johnson syndrome and exfoliative dermatitis. Systemic toxic reactions such as agranulocytosis, acute hemolytic anemia, purpura hemorrhagica, drug fever, jaundice, and contact dermatitis indicate hypersensitivity to sulfonamides. Particular caution should be employed if areas of denuded or abraded skin are involved. FOR EXTERNAL USE ONLY. Keep away from eyes. Keep out of the reach of children. Contents under pressure. Do not puncture or incinerate container. Do not expose to temperatures above 120ºF (49ºC).
PRECAUTIONS
General
The object of this therapy is to achieve desquamation without irritation,
but sodium sulfacetamide and sulfur can cause reddening and scaling of epidermis.
These side effects are not unusual in the treatment of acne vulgaris, but patients
should be cautioned about the possibility. If irritation develops, use of the product
should be discontinued and appropriate therapy instituted. Patients should be
carefully observed for possible local irritation or sensitization during long-term
therapy.
Information for Patients
Avoid contact with eyes, eyelids, lips, and mucous membranes. If accidental contact occurs, rinse with water. If excessive irritation develops discontinue use and consult your physician.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential.
Pregnancy
Category C. Animal reproduction studies have not been conducted with CLARIFOAM EF Emollient Foam. It is also not known whether CLARIFOAM EF Emollient Foam can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. CLARIFOAM EF Emollient Foam should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether sodium sulfacetamide is excreted in the human milk following topical use of CLARIFOAM EF Emollient Foam. However, small amounts of orally administered sulfonamides have been reported to be eliminated in human milk. In view of this and because many drugs are excreted in human milk, caution should be exercised when CLARIFOAM EF Emollient Foam is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in children under the age of 12 have not been established.
ADVERSE REACTIONS
Although rare, sodium sulfacetamide may cause local irritation.
DOSAGE AND ADMINISTRATION
Shake Vigorously, Tap Bottom of Can and Prime Before Initial
Use. Shake Vigorously and Tap Before Each Use.
To Prime: After shaking, gently tap bottom of can onto palm of other hand or a solid
surface at least 3 times. Hold the can upright, direct away from patient, and firmly
depress the actuator for 1 to 3 seconds or until foam begins to dispense. (If foam
does not dispense within 3 seconds: re-shake can, gently tap bottom of can onto a
solid surface at least 3 times, and depress the actuator again until foam begins to
dispense.)
Before Each Use: Cleanse affected skin thoroughly and pat dry before each application. Shake
vigorously and gently tap bottom of can onto palm of other hand or a solid surface at
least 3 times.
During Use: Holding can upright, dispense CLARIFOAM EF Emollient Foam onto the
fingertips.
WASH-OFF APPLICATION: Massage the dispensed foam into the affected
area and
wait 10 minutes. Rinse thoroughly with water and pat dry. Treat the
affected area 1 to 3
times daily, or as directed by a physician.
LEAVE-ON
APPLICATION: Massage the foam into the affected area 1 to 3 times
daily, or as
directed by a physician.
Wipe off any excess foam from actuator after
use.
HOW SUPPLIED
CLARIFOAM EF Emollient Foam is supplied in a 60g (NDC 16781-154-60) aluminum can.
Store between 59° and 86°F (15° and
30°C).
Protect from freezing.
Store upright.
Manufactured For:

Onset
Therapeutics
Cumberland, RI 02864
www.onsettx.com
888-713-8154
PATENT PENDING
P/N 2603-pf Rev. 4
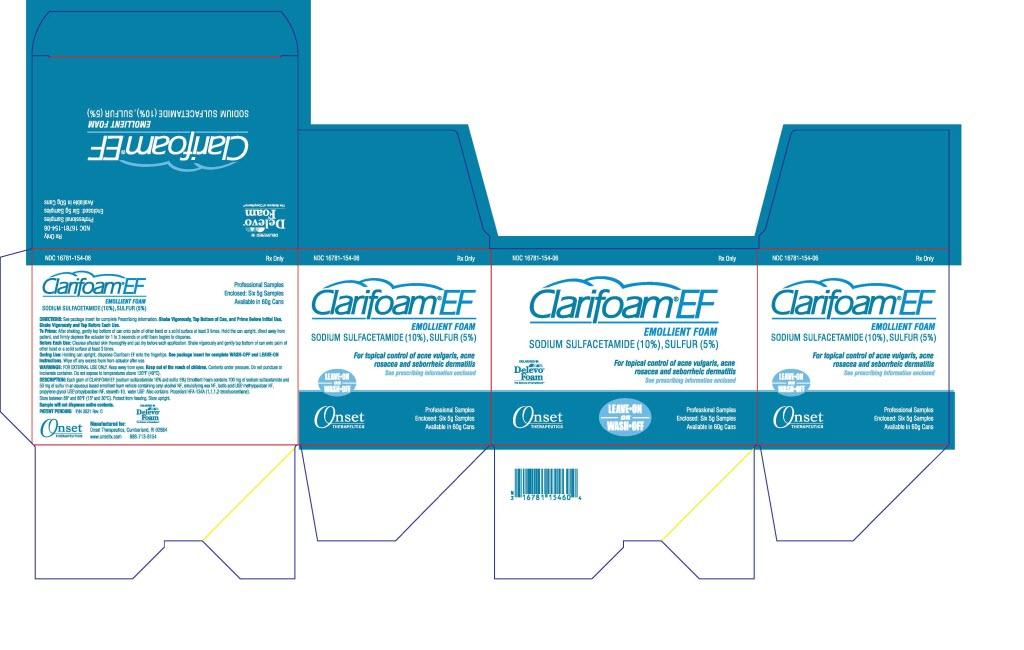
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Outer Package - 5g
NDC 16781-154-06
Rx Only
Clarifoam®™ EF Emollient Foam
Sodium
Sulfactetamide (10%), Sulfur (5%)
For topical control of
acne vulgaris, acne rosacea, and senhorrheic dermatitis
See prescribing
information enclosed
LEAVE-ON
OR
WASH-OFF


PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Inner Label - 5g
NDC 16781-154-06
Rx Only
Professional
Sample
Not for Sale
Net Weight 5g
Clarifoam®™ EF Emollient Foam
Sodium
Sulfactetamide (10%), Sulfur (5%)
LEAVE-ON
OR
WASH-OFF
Sample will not dispense entire
contents.



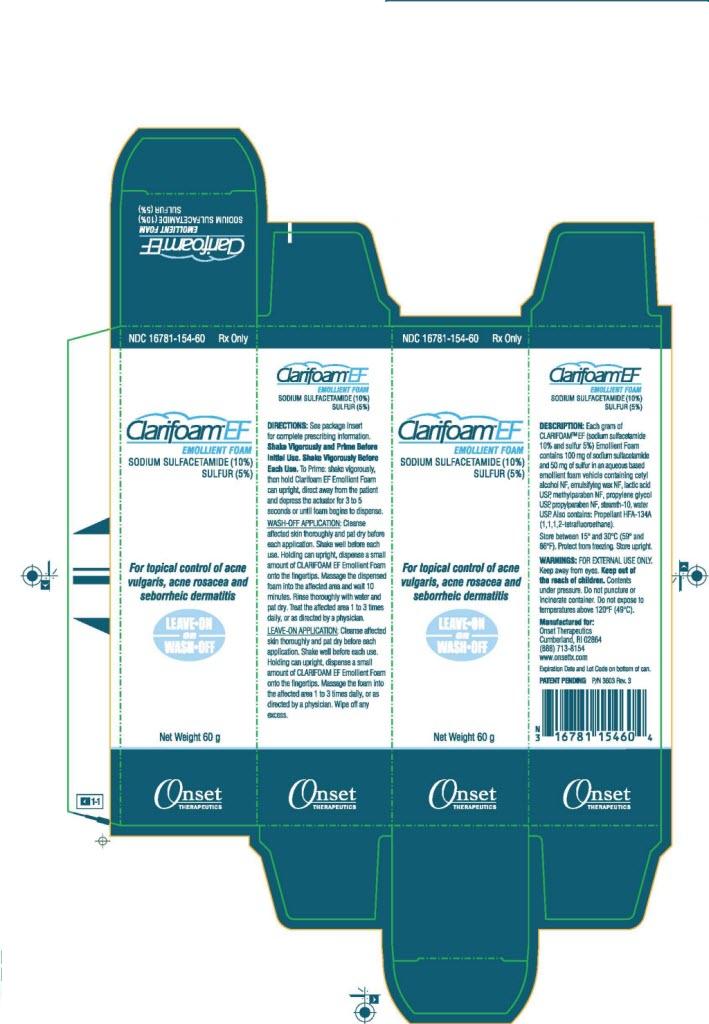
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Outer Package - 60g
NDC 16781-154-60
Rx Only
Clarifoam®™ EF Emollient Foam
Sodium
Sulfactetamide (10%), Sulfur (5%)
For topical control of
acne vulgaris, acne rosacea, and senhorrheic dermatitis
LEAVE-ON
OR
WASH-OFF
Net Weight 60g

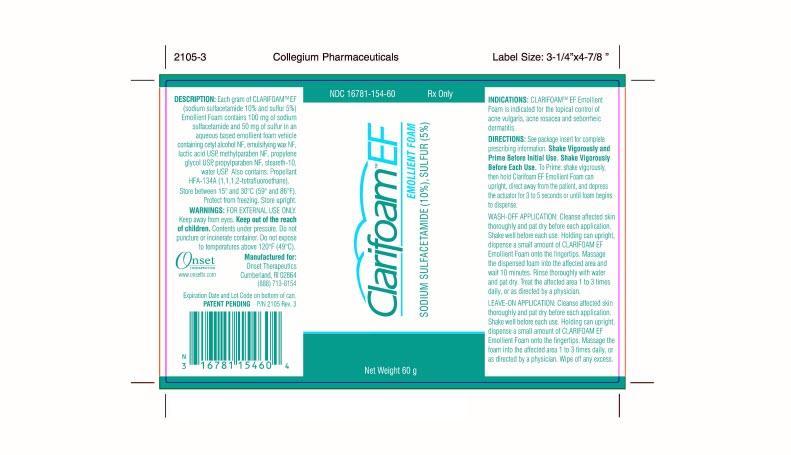
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Inner Label - 60g
NDC 16781-154-60
Rx Only
Clarifoam®™ EF Emollient Foam
Sodium
Sulfactetamide (10%), Sulfur (5%)
Net Weight 60g

| CLARIFOAM EF
sulfur,sulfacetamide sodium aerosol, foam |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 06/01/2007 | ||
| Labeler - Onset Therapeutics, LLC (793223707) |
| Registrant - Onset Therapeutics, LLC (964275155) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Onset Therapeutics, LLC | 793223707 | Manufacture | |