GUAIFENESIN
-
guaifenesin tablet
Prasco Laboratories
----------
Guaifenesin1000 mg
Tablets
11 DESCRIPTION
Each white, scored, sustained-release tablet for oral administration contains:
Guaifenesin………………….1000 mg
Inactive Ingredients: Calcium phosphate diabasic, Eudragit NE 30, magnesium stearate and methylcellulose.
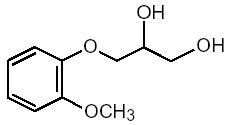
Guaifenesin is an expectorant having the chemical name 3-(2-methoxyphenoxy)-1,2-propanediol with the following structural formula:
C10H14O4. Molecular weight = 198.22
12 CLINICAL PHARMACOLOGY
Guaifenesin is an expectorant which increases respiratory tract fluid secretions and helps to loosen phlegm and bronchial secretions. By reducing the viscosity of secretions, guaifenesin increases the efficiency of the cough reflex and of ciliary action in removing accumulated secretions from the trachea and bronchi. Guaifenesin is readily absorbed from the gastrointestinal tract and is rapidly metabolized and excreted in the urine. Guaifenesin has a plasma half-life of one hour. The major urinary metabolite is b-(2-methoxyphenoxy) lactic acid.
1 INDICATIONS & USAGE
Guaifenesin tablets are indicated for the temporary relief of coughs associated with respiratory tract infections and related conditions such as sinusitis, pharyngitis, bronchitis, and asthma, when these conditions are complicated by tenacious mucus and/or mucus plugs and congestion. The drug is effective in productive as well as non-productive cough, but is of particular value in dry, non-productive cough which tends to injure the mucous membrane of the air passages.
4 CONTRAINDICATIONS
The drug is contraindicated in patients with hypersensitivity to guaifenesin.
PRECAUTIONS
GENERAL PRECAUTIONS
Before prescribing medication to suppress or modify cough, it is important to ascertain that the underlying cause of cough is identified, that modification of cough does not increase the risk of clinical or physiological complications, and that appropriate therapy for the primary disease is instituted.
DRUG & OR LABORATORY TEST INTERACTIONS
Guaifenesin may increase renal clearance for the urate and thereby lower serum uric acid levels. Guaifenesin may produce an increase in urinary 5-hydroxyindoleacetic acid and may therefore interfere with the interpretation of this test for the diagnosis of carcinoid syndrome. It may also falsely elevate the VMA test for catechols. Administration of this drug should be discontinued 48 hours prior to the collection of urine specimens for such tests.
1 CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
No data are available on the long-term potential for carcinogenesis, mutagenesis, or impairment of fertility in animals or humans.
1 PREGNANCY
Category C: Animal reproduction studies have not been conducted with guaifenesin. It is also not known whether guaifenesin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Guaifenesin should be given to a pregnant woman only if clearly needed.
3 NURSING MOTHERS
It is not known whether guaifenesin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when guaifenesin is administered to a nursing woman and a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
6 ADVERSE REACTIONS
No serious side effects from guaifenesin have been reported.
10 OVERDOSAGE
Overdosage with guaifenesin is unlikely to produce toxic effects since its toxicity is low. Guaifenesin, when administered by stomach tube to test animals in doses up to 5 grams/kg produced no signs of toxicity. In severe cases of overdosage, treatment should be aimed at reducing further absorption of the drug. Gastric emptying (Syrup of Ipecac) and/or lavage is recommended as soon as possible after ingestion.
.
2 DOSAGE & ADMINISTRATION
Adults and children over 12 years of age: One tablet every 12 hours, not to exceed 2 tablets in 24 hours.
Children 6 to 12 years of age: ½ tablet every 12 hours, not to exceed 1 tablet in 24 hours.
16 HOW SUPPLIED
Bottles of 100 scored dye-free tablets imprinted with “322” on one side and the Prasco Logo on the other side. NDC 66993-322-02.
Store at room temperature between 15°- 30 °C (59°-86°F). Dispense in tight, light resistant container with a child resistant closure.
Rx Only
Manufactured for:
Prasco Laboratories
Cincinnati, Ohio 45249
By:
Sovereign Pharmaceuticals, Ltd.
Fort Worth, Texas 76118
PIPRAS-14 Iss. 6/2002
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

| GUAIFENESIN
guaifenesin tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 12/01/2010 | 01/20/2011 | |
| Labeler - Prasco Laboratories (065969375) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sovereign Pharmaceuticals, Ltd. | 623168267 | MANUFACTURE | |