COLD REMEDY
-
zinc acetate and
zinc gluconate tablet, orally disintegrating
Meijer Distribution Inc
----------
Meijer Cold Remedy Drug FactsActive ingredient
Zincum Aceticum 2x
Zincum Gluconicum 1x
Purpose
Reduces duration of the common cold
Uses
- reduces duration of the common cold
- may reduce the severity of cold symptoms:
- sore throat
- stuffy nose
- sneezing
- coughing
- congestion
Warnings
Stop use and ask a doctor if
symptoms persist or are accompanied by fever.
This product was formulated to shorten the duration of the common cold and may not be effective for flu or allergies.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
- For best results, use at the first sign of a cold and continue to use for an additional 48 hours after symptoms subside.
- Adults and children 3 years of age and older:
- Dissolve entire tablet in mouth. Do not chew. Do not swallow whole.
- Take one tablet at the onset of symptoms.
- Repeat every three hours until symptoms are gone.
- To avoid minor stomach upset, do not take on an empty stomach.
- Do not eat or drink for 15 minutes after use. Do not eat or drink citrus fruits or juices for 30 minutes before or after use. Otherwise, drink plenty of fluids.
- Children under 3 years of age: Consult a doctor before use.
Other information
- store at 20º-25ºC (68º-77ºF)
Inactive ingredients
crospovidone, dextrates, flavors, magnesium stearate, polyethylene glycol, sodium lauryl sulfate, sodium starch glycolate, sucralose
Questions or comments?
1-800-719-9260
Principal Display Panel
Melteez®
Melts in your Mouth
Cold Remedy
Zincum Aceticum, Zincum Gluconicum
Homeopathic
Reduces the Duration of the Common Cold
May Reduce the Severity of Cold Symptoms
Cherry
Compare to Zicam® Cold Remedy active ingredients
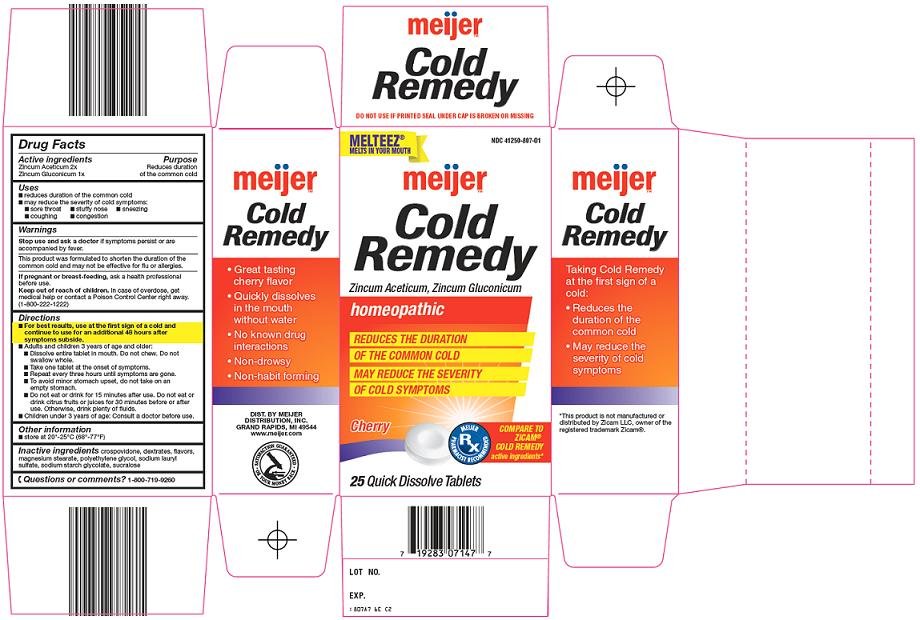
Cold Remedy Carton
| COLD REMEDY
zincum aceticum, zincum gluconicum tablet, orally disintegrating |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved homeopathic | 08/06/2008 | ||
| Labeler - Meijer Distribution Inc (006959555) |