DRY EYE RELIEF
-
atropa belladonna,
euphrasia stricta and
mercuric chloride solution/ drops
Similasan AG
----------
Similasan Dry Eye Relief Preservative FreeActive Ingredient
Belladonna* 6X
Enter section text here
*containing 0.000002% alkaloids calculated as hyoscyamine.
Purpose
dryness, redness
Active Ingredient
Euphrasia 6X
Purpose
redness, inflammation
Active Ingredient
Mercurius sublimatus 6X
Purpose
dryness
Uses
According to homeopathic principles, the active ingredients in this medication temporarily minor symptoms such as:
- dry eye
- redness of eyes and lids
- reflex watering secondary to dry eye
- sensation of grittiness
- sensitivity to light
Warnings
- For external use only.
- Initial exacerbation of symptoms may occur.
- Use only if single-use dropper is intact.
- To avoid contamination, do not touch the tip of the dropper to any surface. Do not reuse. Once opened, discard.
- Contact wearers: consult a physician prior to using.
Do not use:
- if the solution changes color or becomes cloudy
Stop use and ask a doctor if:
- symptoms worsen or persist for more than 72 hours.
- changes in vision occur.
- you experience eye pain.
If pregnant or breast feeding,
ask a health professional before use.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
For adults and children age 2 and over:
- remove a single use dropper
- twist flat end with ball to remove
- squeeze plastic tip to release 2-3 drops into eye and discard applicator
- apply as needed throughout the day or night
- use a dropper for every application
Other information
Active ingredients are manufactured according to homeopathic principles and are therefore non-toxic and have no known side effects.
Inactive Ingredients
Phosphate buffer, Purified water
Questions?
Reach our representatives, M-F, 8am-5pm (MT), or our 24 hour recorded product information at 1-800-240-9780. Visit us: www.SimilasanUSA.com
Principal Display Panel
Homeopathic
NDC 59262-351-12
Similasan
Dry Eye Relief
Preservative Free
0.45 ml/ 0.015 fl oz each
20 Sterile Single-Use Droppers
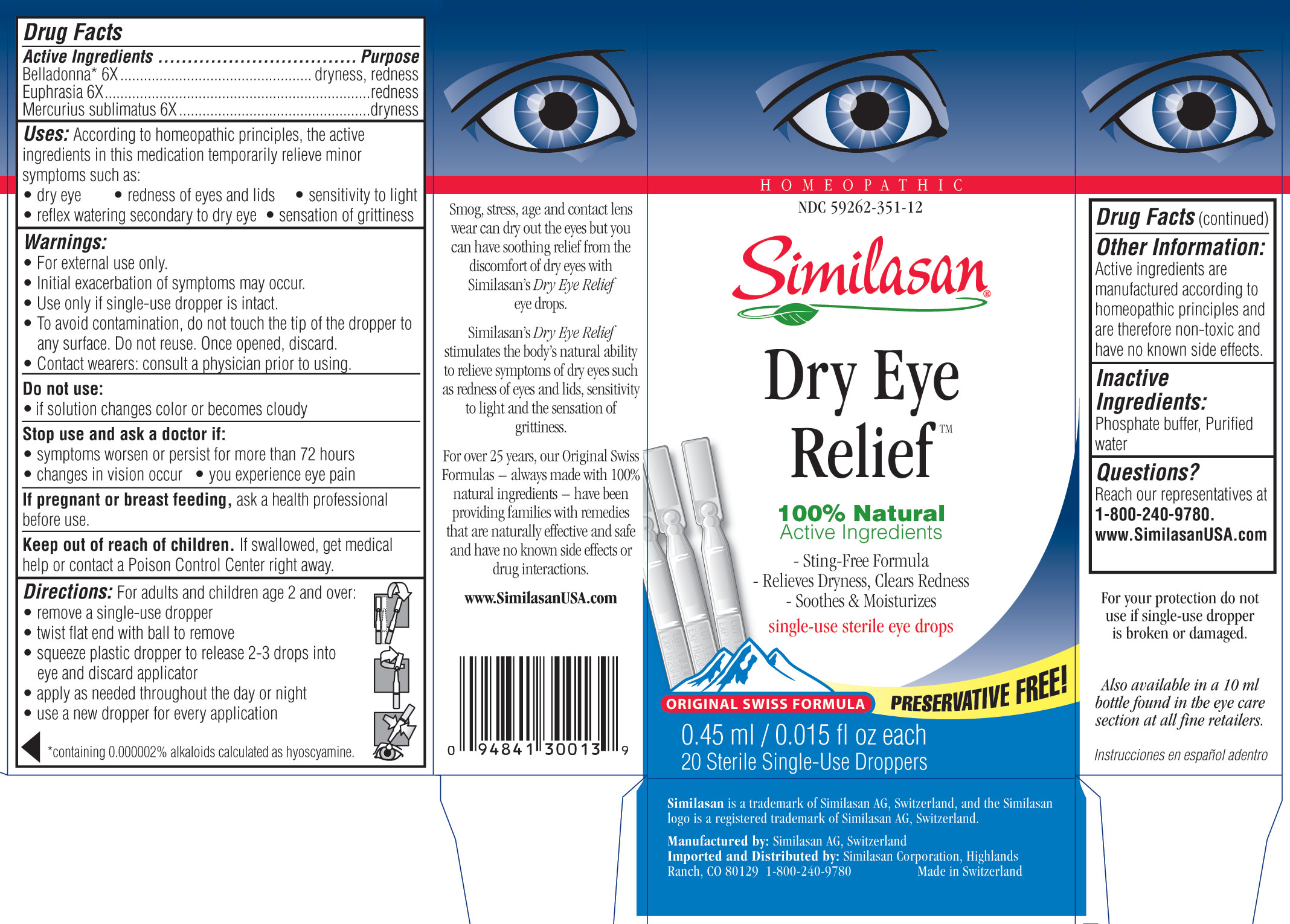
Dry Eye Preservative Free Box
| DRY EYE RELIEF
belladonna 6x, euphrasia 6x, mercurius sublimatus 6x solution/ drops |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED HOMEOPATHIC | 01/01/1985 | ||
| Labeler - Similasan AG (481545754) |