azilect (rasagiline mesylate) tablet
[Teva Pharmaceutical Industries, LTD.]
DESCRIPTION
AZILECT® Tablets contain rasagiline (as the mesylate), a propargylamine-based drug indicated for the treatment of idiopathic Parkinson’s disease. It is designated chemically as: 1H-Inden-1-amine, 2, 3-dihydro-N-2-propynyl-, (1R)-, methanesulfonate. The empirical formula of rasagiline mesylate is (C12H13N)CH4SO3 and its molecular weight is 267.34.
Its structural formula is:
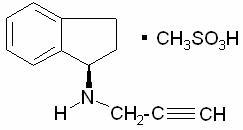
Rasagiline mesylate is a white to off-white powder, freely soluble in water or ethanol and sparingly soluble in isopropanol. Each AZILECT tablet for oral administration contains rasagiline mesylate equivalent to 0.5 mg or 1 mg of rasagiline base.
Each AZILECT tablet also contains the following inactive ingredients: mannitol, starch, pregelatinized starch, colloidal silicon dioxide, stearic acid and talc.
CLINICAL PHARMACOLOGY
Mechanism of Action
AZILECT is an irreversible monoamine oxidase inhibitor indicated for the treatment of idiopathic Parkinson’s disease. AZILECT inhibits MAO type B, but adequate studies to establish whether rasagiline is selective for MAO type B (MAO-B) in humans have not yet been conducted.
MAO, a flavin-containing enzyme, is classified into two major molecular species, A and B, and is localized in mitochondrial membranes throughout the body in nerve terminals, brain, liver and intestinal mucosa. MAO regulates the metabolic degradation of catecholamines and serotonin in the CNS and peripheral tissues. MAO-B is the major form in the human brain. In ex vivo animal studies in brain, liver and intestinal tissues, rasagiline was shown to be a potent, irreversible monoamine oxidase type B (MAO-B) selective inhibitor. Rasagiline at the recommended therapeutic dose was also shown to be a potent and irreversible inhibitor of MAO-B in platelets. The selectivity of rasagiline for inhibiting only MAO-B (and not MAO-A) in humans and the sensitivity to tyramine during rasagiline treatment at any dose has not been sufficiently characterized to avoid restriction of dietary tyramine and amines contained in medications. (See WARNINGS).
The precise mechanisms of action of rasagiline are unknown. One mechanism is believed to be related to its MAO-B inhibitory activity, which causes an increase in extracellular levels of dopamine in the striatum. The elevated dopamine level and subsequent increased dopaminergic activity are likely to mediate rasagiline’s beneficial effects seen in models of dopaminergic motor dysfunction.
Pharmacodynamics
Platelet MAO Activity in Clinical Studies: Studies in healthy subjects and in Parkinson’s disease patients have shown that rasagiline inhibits platelet MAO-B irreversibly. The inhibition lasts at least 1 week after last dose. Almost 25-35% MAO-B inhibition was achieved after a single rasagiline dose of 1 mg/day and more than 55% of MAO-B inhibition was achieved after a single rasagiline dose of 2 mg/day. Over 90% inhibition was achieved 3 days after rasagiline daily dosing at 2 mg/day and this inhibition level was maintained 3 days post-dose. Multiple doses of rasagiline of 0.5, 1 and 2 mg per day resulted in complete MAO-B inhibition.
Pharmacokinetics
Rasagiline’s pharmacokinetics are linear with doses over the range of 1 -10 mg. Its mean steady-state half life is 3 hours but there is no correlation of pharmacokinetics with its pharmacological effect because of its irreversible inhibition of MAO-B.
Absorption
Rasagiline is rapidly absorbed, reaching peak plasma concentration (Cmax) in approximately 1 hour. The absolute bioavailability of rasagiline is about 36%.
Food does not affect the Tmax of rasagiline, although Cmax and exposure (AUC) are decreased by approximately 60% and 20%, respectively, when the drug is taken with a high fat meal. Because AUC is not significantly affected, AZILECT can be administered with or without food (See DOSAGE AND ADMINISTRATION).
Distribution
The mean volume of distribution at steady-state is 87 L, indicating that the tissue binding of rasagiline is in excess of plasma protein binding. Plasma protein binding ranges from 88-94% with mean extent of binding of 61-63% to human albumin over the concentration range of 1-100 ng/mL.
Metabolism and Elimination
Rasagiline undergoes almost complete biotransformation in the liver prior to excretion. The metabolism of rasagiline proceeds through two main pathways: N-dealkylation and/or hydroxylation to yield 1-aminoindan (AI), 3-hydroxy-N-propargyl-1 aminoindan (3-OH-PAI) and 3-hydroxy-1-aminoindan (3-OH-AI). In vitro experiments indicate that both routes of rasagiline metabolism are dependent on the cytochrome P450 (CYP) system, with CYP1A2 being the major isoenzyme involved in rasagiline metabolism. Glucuronide conjugation of rasagiline and its metabolites, with subsequent urinary excretion, is the major elimination pathway.
After oral administration of 14C-labeled rasagiline, elimination occurred primarily via urine and secondarily via feces (62% of total dose in urine and 7% of total dose in feces over 7 days), with a total calculated recovery of 84% of the dose over a period of 38 days. Less than 1% of rasagiline was excreted as unchanged drug in urine.
Special Populations
Hepatic Insufficiency
Following repeat dose administration (7 days) of rasagiline (1 mg/day) in subjects with mild hepatic impairment (Child-Pugh score 5-6), AUC and Cmax were increased by 2 fold and 1.4 fold, respectively, compared to healthy subjects. In subjects with moderate hepatic impairment (Child-Pugh score 7-9), AUC and Cmax were increased by 7 fold and 2 fold, respectively, compared to healthy subjects. (See WARNINGS, Hepatic Insufficiency and DOSAGE AND ADMINISTRATION, Patients with Hepatic Impairment).
Renal Insufficiency
Conclusive data are not available for renally impaired patients. As unconjugated rasagiline is not excreted by the kidney, rasagiline can be given at usual doses in patients with mild renal impairment.
Geriatric
Since age has little influence on rasagiline pharmacokinetics, it can be administered at the recommended dose in the elderly.
Pediatric
AZILECT has not been investigated in patients below 18 years of age.
Gender
The pharmacokinetic profile of rasagiline is similar in men and women.
Drug-Drug Interactions
Tyramine Effect
(See WARNINGS, PRECAUTIONS-Information for Patients, OVERDOSE, and DOSAGE AND ADMINISTRATION).
Levodopa
Data from population pharmacokinetic studies comparing rasagiline clearance in the presence and absence of levodopa have given conflicting results. Although there may be some increase in rasagiline blood levels in the presence of levodopa, the effect is modest and rasagiline dosing need not be modified in the presence of levodopa.
Effect of Other Drugs on the Metabolism of AZILECT
In vitro metabolism studies showed that CYP 1A2 was the major enzyme responsible for the metabolism of rasagiline. There is the potential for inhibitors of this enzyme to alter AZILECT clearance when coadministered. (See WARNINGS, Ciprofloxacin and Other CYP1A2 Inhibitors and DOSAGE AND ADMINISTRATION, Patients Taking Ciprofloxacin and Other CYP1A2 Inhibitors).
Ciprofloxacin
When ciprofloxacin, an inhibitor of CYP 1A2, was administered to healthy volunteers (n=12) at 500 mg (BID) with rasagiline at 2 mg/day, the AUC of rasagiline increased by 83% and there was no change in the elimination half life. (See WARNINGS, Ciprofloxacin and Other CYP1A2 Inhibitors and DOSAGE AND ADMINISTRATION, Patients Taking Ciprofloxacin and Other CYP1A2 Inhibitors).
Theophylline
Coadministration of rasagiline 1 mg/day and theophylline, a substrate of CYP1A2, up to 500 mg twice daily to healthy subjects (n=24) did not affect the pharmacokinetics of either drug.
Antidepressants
Severe CNS toxicity associated with hyperpyrexia and death has been reported with the combination of tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), or serotonin-norepinephrine reuptake inhibitors (SNRIs) and non-selective MAOIs or selective MAO-B inhibitors. (See WARNINGS, Coadministration with Antidepressants).
Effect of AZILECT on Other Drugs
No additional in vivo trials have investigated the effect of AZILECT on other drugs metabolized by the cytochrome P450 enzyme system. In vitro studies showed that rasagiline at a concentration of 1ug/ml (equivalent to a level that is 160 times the average Cmax ~ 5.9-8.5 ng/mL in Parkinson’s disease patients after 1 mg rasagiline multiple dosing) did not inhibit cytochrome P450 isoenzymes, CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP3A4 and CYP4A. These results indicate that rasagiline is unlikely to cause any clinically significant interference with substrates of these enzymes.
CLINICAL TRIALS
The effectiveness of AZILECT (rasagiline tablets) for the treatment of Parkinson’s disease was established in three 18- to 26-week, randomized, placebo-controlled trials. In one of these trials AZILECT was given as initial monotherapy and in the other two as adjunctive therapy to levodopa.
Monotherapy Use of AZILECT
The monotherapy trial was a double-blind, randomized, fixed-dose parallel group, 26-week study in early Parkinson’s disease patients not receiving any concomitant dopaminergic therapy at the start of the study. The majority of the patients were not treated with any anti-Parkinson’s disease medication before receiving rasagiline treatment.
In this trial, 404 patients were randomly assigned to receive placebo (138 patients), rasagiline 1 mg/day (134 patients) or rasagiline 2 mg/day (132 patients). Patients were not allowed to take levodopa, dopamine agonists, selegiline or amantadine, but if necessary, could take stable doses of anticholinergic medication. The average Parkinson’s disease duration was approximately 1 year (range 0 to 11 years).
The primary measure of effectiveness was the change from baseline in the total score of the Unified Parkinson’s Disease Rating Scale (UPDRS), [mentation (Part I) + activities of daily living (ADL) (Part II) + motor function (Part III)]. The UPDRS is a multi-item rating scale that measures the ability of a patient to perform mental and motor tasks as well as activities of daily living. A reduction in the score represents improvement and a beneficial change from baseline appears as a negative number.
Rasagiline (1 or 2 mg once daily) had a significant beneficial effect relative to placebo on the primary measure of effectiveness in patients receiving six months of treatment and not on dopaminergic therapy. Patients who received rasagiline had significantly less worsening in the UPDRS score, compared to those who received placebo. The effectiveness of rasagiline 1 mg and 2 mg was comparable. Table 1 displays the results of the monotherapy trial.
| Primary Measure of Effectiveness: Change in total UPDRS score | |||
| Change from | |||
| Baseline score | baseline to | p-value vs. | |
| termination score | placebo | ||
| Placebo | 24.5 | 3.9 | --- |
| 1.0 mg/day | 24.7 | 0.1 | 0.0001 |
| 2.0 mg/day | 25.9 | 0.7 | 0.0001 |
For the comparison between rasagiline 1mg/day and placebo, no differences in effectiveness based on age or gender were detected.
Adjunctive Use of AZILECT
Two multicenter, randomized, multinational trials were conducted in more advanced Parkinson’s disease patients treated chronically with levodopa and experiencing motor fluctuations (including but not limited to, end of dose “wearing off,” sudden or random “off,” etc.). The first (Study 1) was conducted in North America (U.S. and Canada) and compared two doses (0.5 mg and 1 mg daily) of rasagiline and placebo while the second (Study 2) was conducted outside of North America (several European countries, Argentina, Israel) and studied only a single dose (1 mg daily) of rasagiline and placebo. Patients had had Parkinson’s disease for an average of 9 years (range 5 months to 33 years), had been taking levodopa for an average of 8 years (range 5 months to 32 years), and had been experiencing motor fluctuations for approximately 3 to 4 years (range 1 month to 23 years). Patients kept home diaries just prior to baseline and at specified intervals during the trial. Diaries recorded one of the following four conditions for each half-hour interval over a 24-hour period: “ON” (period of relatively good function and mobility) as either “ON” with no dyskinesia or without troublesome dyskinesia, or “ON” with troublesome dyskinesia, “OFF” (period of relatively poor function and mobility) or asleep. “Troublesome” dyskinesia is defined as that which interferes with the patient’s daily activity. All patients had been inadequately controlled and were experiencing motor fluctuations typical of advanced stage disease despite receiving levodopa/decarboxylase inhibitor. The average dose of levodopa/decarboxylase inhibitor was approximately 700 to 800 mg (range 150 to 3000 mg/day). Patients were also allowed to take stable doses of additional anti-PD medications at entry into the trials. In both trials, approximately 65% of patients were on dopamine agonists and in the North American study (Study 1) approximately 35% were on entacapone. The majority of patients taking entacapone were taking a dopamine agonist as well.
In both trials the primary measure of effectiveness was the change in the mean number of hours that were spent in the “OFF” state at baseline compared to the mean number of hours that were spent in the “OFF” state during the treatment period.
The first adjunct study (Study 1) was a double-blind, randomized, fixed-dose, parallel group trial conducted in 472 levodopa-treated Parkinson’s disease patients who were experiencing motor fluctuations. Patients were randomly assigned to receive placebo (159 patients), rasagiline 0.5 mg/day (164 patients), or rasagiline 1 mg/day (149 patients), and were treated for 26 weeks. Patients averaged approximately 6 hours daily in the “OFF” state at baseline, as confirmed by home diaries.
The second adjunct study (Study 2) was a double-blind, randomized, parallel group trial conducted in 687 levodopa-treated Parkinson’s disease patients who were experiencing motor fluctuations. Patients were randomly assigned to receive placebo (229 patients), rasagiline 1 mg/day (231 patients) or an active comparator, a COMT inhibitor taken along with scheduled doses of levodopa/decarboxylase inhibitor (227 patients). Patients were treated for 18 weeks. Patients averaged approximately 5.6 hours daily in the “OFF” state at baseline as confirmed by home diaries.
In both studies rasagiline 1 mg once daily reduced “OFF” time compared to placebo when added to levodopa in patients experiencing motor fluctuations (Tables 2 and 3). The lower dose (0.5 mg) of rasagiline also significantly reduced “OFF” time (Table 2), but had a numerically smaller effect than the 1 mg dose of rasagiline. In Study 2, the active comparator also reduced “OFF” time when compared to placebo.
| Primary Measure of Effectiveness: Change in mean total daily “OFF” time | |||
| Change from | p-value vs. | ||
| Baseline | baseline to treatment | placebo | |
| (hours) | period (hours) | ||
| Placebo | 6.0 | -0.9 | --- |
| 0.5 mg/day | 6.0 | -1.4 | 0.0199 |
| 1.0 mg/day | 6.3 | -1.9 | < 0.0001 |
| Primary Measure of Effectiveness: Change in mean total daily “OFF” time | |||
| Change from baseline | p-value vs. | ||
| Baseline | to treatment period | placebo | |
| (hours) | (hours) | ||
| Placebo | 5.5 | - 0.40 | --- |
| 1.0 mg/day | 5.6 | -1.2 | 0.0001 |
In both studies, dosage reduction of levodopa was allowed within the first 6 weeks if dopaminergic side effects, including dyskinesia and hallucinations, emerged. In Study 1, levodopa dosage reduction occurred in 8% of patients in the placebo group and in 16% and 17% of patients in the 0.5 mg/day and 1 mg/day rasagiline groups, respectively. In those patients who had levodopa dosage reduced, the dose was reduced on average by about 7%, 9%, and 13% in the placebo, 0.5 mg/day, and 1 mg/day groups, respectively. In Study 2, levodopa dosage reduction occurred in 6% of patients in the placebo group and in 9% in the rasagiline 1 mg/day group. In patients who had their levodopa dosage reduced, the dose was reduced on average by about 13% and 11% in the placebo and the rasagiline groups, respectively.
For the comparison between rasagiline 1 mg/day and placebo in both studies, no differences in effectiveness based on age or gender were detected.
Several secondary outcome assessments in the two studies showed statistically significant improvements with rasagiline. These included effects on the activities of daily living (ADL) subscale of the UPDRS performed during an “OFF” period and the motor subscale of the UPDRS performed during an “ON” period. In both scales, a negative response represents improvement. Tables 4 and 5 show these results for Studies 1 and 2.
| Baseline | Change from baseline to last | |
| (score) | value | |
| UPDRS ADL (Activities of Daily Living) subscale score while “OFF” | ||
| Placebo | 15.5 | 0.68 |
| 0.5 mg/day | 15.8 | -0.60 |
| 1.0 mg/day | 15.5 | -0.68 |
| UPDRS Motor subscale score while “ON” | ||
| Placebo | 20.8 | 1.21 |
| 0.5 mg/day | 21.5 | -1.43 |
| 1.0 mg/day | 20.9 | -1.30 |
| Baseline | Change from baseline | |
| (score) | to last value | |
| UPDRS ADL (Activities of Daily Living) subscale score while “OFF” | ||
| Placebo | 18.7 | -0.89 |
| 1.0 mg/day | 19.0 | -2.61 |
| UPDRS Motor subscale score while “ON” | ||
| Placebo | 23.5 | -0.82 |
| 1.0 mg/day | 23.8 | -3.87 |
INDICATIONS AND USAGE
AZILECT (rasagiline tablets) is indicated for the treatment of the signs and symptoms of idiopathic Parkinson’s disease as initial monotherapy and as adjunct therapy to levodopa.
The effectiveness of AZILECT was demonstrated in patients with early Parkinson’s disease who were receiving AZILECT as monotherapy and who were not receiving any concomitant dopaminergic therapy. The effectiveness of AZILECT as adjunct therapy was demonstrated in patients with Parkinson’s disease who were treated with levodopa.
CONTRAINDICATIONS
Meperidine and Other Analgesics
AZILECT is contraindicated for use with meperidine. Serious reactions have been precipitated with concomitant use of meperidine (e.g., Demerol and other tradenames) and MAO inhibitors including selective MAO-B inhibitors. These reactions have been characterized by coma, severe hypertension or hypotension, severe respiratory depression, convulsions, malignant hyperpyrexia, excitation, peripheral vascular collapse and death. At least 14 days should elapse between discontinuation of AZILECT and initiation of treatment with meperidine.
For similar reasons, AZILECT should not be administered with the analgesic agents tramadol, methadone, and propoxyphene.
Other Drugs
AZILECT should not be used with the antitussive agent dextromethorphan. The combination of MAO inhibitors and dextromethorphan has been reported to cause brief episodes of psychosis or bizarre behavior. AZILECT is also contraindicated for use with St. John’s wort, mirtazapine (a tetracyclic antidepressant), and cyclobenzaprine (a tricyclic muscle relaxant).
Sympathomimetic Amines
Like other MAOIs, AZILECT is contraindicated for use with sympathomimetic amines, including amphetamines as well as cold products and weight-reducing preparations that contain vasoconstrictors (e.g., pseudoephedrine, phenylephrine, phenylpropanolamine, and ephedrine). Severe hypertensive reactions have followed the administrations of sympathomimetics and non-selective MAO inhibitors. At least one case of hypertensive crisis has been reported in a patient taking the recommended doses of a selective MAO-B inhibitor and a sympathomimetic medication (ephedrine).
MAO Inhibitors
AZILECT should not be administered along with other MAO inhibitors because of the increased risk of non-selective MAO inhibition that may lead to a hypertensive crisis. At least 14 days should elapse between discontinuation of AZILECT and initiation of treatment with MAO inhibitors.
Surgery
As with other MAOIs, patients taking AZILECT should not undergo elective surgery requiring general anesthesia. Also, they should not be given cocaine or local anesthesia containing sympathomimetic vasoconstrictors. AZILECT should be discontinued at least 14 days prior to elective surgery. If surgery is necessary sooner, benzodiazepines, mivacurium, rapacuronium, fentanyl, morphine, and codeine may be used cautiously.
Pheochromocytoma
As with other MAOIs, AZILECT is contraindicated in patients with pheochromocytoma.
WARNINGS
Need for Restriction of Dietary Tyramine and Amines Contained in Medications
AZILECT treatment at any dose may be associated with a hypertensive crisis/“cheese reaction” if the patient ingests tyramine-rich foods, beverages, or dietary supplements or amines (from over-the-counter medications). Hypertensive crisis, which in some cases may be fatal, consists of marked systemic blood pressure elevation and requires immediate treatment/hospitalization.
MAO in the gastrointestinal tract and liver (primarily type A) is thought to provide vital protection from exogenous amines (e.g., tyramine) that have the capacity, if absorbed intact, to cause a hypertensive crisis the so-called “cheese reaction.” If significant amounts of certain exogenous amines gain access to the systemic circulation – e.g., tyramine from fermented cheese, red wine, herring, or amines contained in over-the-counter cough/cold medications – they can cause release of norepinephrine, which may significantly increase systemic blood pressure. MAO inhibitors that selectively inhibit MAO-B are generally devoid of the potential to cause a hypertensive crisis/“cheese reaction” at defined relatively low doses at which tyramine sensitivity has been characterized. The selectivity of rasagiline for inhibiting MAO-B (and not MAO-A) in humans has not been sufficiently characterized to permit rasagiline treatment without restriction of dietary tyramine or amines contained in medications. Even for “selective” MAO-B inhibitors, the selectivity for inhibiting MAO-B typically diminishes and is ultimately lost as the dose is increased beyond particular dose levels.
Patients receiving rasagiline should be instructed about the tyramine content of foods and beverages(see table below) and amine containing medications that should be avoided. Sympathomimetic amines found in over-the-counter medicines to be avoided include pseudoephedrine, phenylephrine, phenylpropanolamine, and ephedrine.
It is also necessary to maintain this dietary tyramine restriction and avoidance of exogenous amines contained in medications for 2 weeks following discontinuation of rasagiline because of the irreversible inhibition of the MAO enzyme and the need for new MAO enzyme synthesis.
Patients should also be instructed about the signs and symptoms of marked blood pressure elevation that could represent a hypertensive emergency requiring immediate treatment/hospitalization. These include severe headache, blurred vision/visual disturbances, difficulty thinking, stupor/coma, seizures, chest pain, unexplained nausea or vomiting, or signs or symptoms of a stroke.
Patients should be told to immediately contact a medical provider to report any severe headache or other atypical or unusual symptoms not previously experienced that could be due to a hypertensive crisis. (See PRECAUTIONS-Information for Patients, OVERDOSE, DOSAGE AND ADMINISTRATION).
| Class of Food or | Tyramine-rich Foods and Beverages to | Acceptable Foods, Containing No or |
| Beverage | Avoid | Little Tyramine |
| Adapted from K. I. Shulman, S.E. Walker, Psychiatric Annals 2001; 31:378-384 | ||
| Meat, Poultry and Fish | Air dried, aged and fermented meats, sausages and salamis (including cacciatore, hard salami and mortadella); pickled herring; and any spoiled or improperly stored meat, poultry and fish (e.g., foods that have undergone changes in coloration, odor, or become moldy); spoiled or improperly stored animal livers | Little Tyramine Fresh meat, poultry and fish, including fresh processed meats (e.g. lunch meats, hot dogs, breakfast sausage, and cooked sliced ham) |
| Vegetables | Broad bean pods (fava bean pods) | All other vegetables |
| Dairy | Aged cheeses | Processed cheeses, mozzarella, ricotta cheese, cottage cheese and yogurt |
| Beverages | All varieties of tap beer and beers that have not been pasteurized so as to allow for ongoing fermentation, red wines | Bottled and canned beers and white wines contain little or no tyramine |
| Miscellaneous | Concentrated yeast extract (e.g., Marmite), sauerkraut, most soybean products (including soy sauce and tofu), OTC supplements containing tyramine | Brewer’s yeast, baker’s yeast, soy milk, commercial chain restaurant pizzas prepared with cheeses low in tyramine |
Coadministration with Antidepressants
Severe CNS toxicity associated with hyperpyrexia and death has been reported with the combination of tricyclic antidepressants and non-selective MAOIs (e.g., Nardil, Parnate) or a selective MAO-B inhibitor, selegiline (Eldepryl). These adverse events have included behavioral and mental status changes, diaphoresis, muscular rigidity, hypertension, syncope and death.
Serious, sometimes fatal, reactions with signs and symptoms including hyperthermia, rigidity, myoclonus, autonomic instability with rapid vital sign fluctuations, and mental status changes progressing to extreme agitation, delirium, and coma have been reported in patients receiving a combination of selective serotonin reuptake inhibitors (SSRIs), including fluoxetine (Prozac), fluvoxamine (Luvox), sertraline (Zoloft), and paroxetine (Paxil) and non-selective MAOIs or the selective MAO-B inhibitor selegiline. Similar reactions have been reported with serotonin-norepinephrine reuptake inhibitors (SNRIs) and non-selective MAOIs or the selective MAO-B inhibitor selegiline.
AZILECT clinical trials did not allow concomitant use of fluoxetine or fluvoxamine with AZILECT, but the following antidepressants and doses were allowed in the AZILECT trials: amitriptyline ≤ 50 mg/daily, trazodone ≤ 100 mg/daily, citalopram ≤ 20 mg/daily, sertraline ≤ 100 mg/daily and paroxetine ≤ 30 mg/daily.
Although a small number of rasagiline-treated patients were concomitantly exposed to antidepressants (tricyclics n= 115; SSRIs n= 141), the exposure, both in dose and number of subjects, was not adequate to rule out the possibility of an untoward reaction from combining these agents. Furthermore, because the mechanisms of these reactions are not fully understood, it seems prudent, in general, to avoid the combination of AZILECT with tricyclic, SSRI, or SNRI (serotonin-norepinephrine reuptake inhibitor) antidepressants. At least 14 days should elapse between discontinuation of AZILECT and initiation of treatment with a tricyclic, SSRI, or SNRI antidepressant. Because of the long half lives of fluoxetine and its active metabolite, at least five weeks (perhaps longer, especially if fluoxetine has been prescribed chronically and/or at higher doses) should elapse between discontinuation of fluoxetine and initiation of AZILECT. (See PRECAUTIONS, Drug Interactions, Selective Serotonin ReuptakeIinhibitors (SSRIs), Tricyclic and Tetracyclic Antidepressants).
Ciprofloxacin and Other CYP1A2 Inhibitors
Rasagiline plasma concentrations may increase up to 2 fold in patients using concomitant ciprofloxacin and other CYP1A2 inhibitors. (See CLINICAL PHARMACOLOGY, Drug-Drug Interactions and DOSAGE AND ADMINISTRATION, Patients Taking Ciprofloxacin and Other CYP1A2 Inhibitors).
Hepatic Insufficiency
Rasagiline plasma concentration may increase in patients with mild (up to 2 fold, Child-Pugh score 5-6), moderate (up to 7 fold, Child-Pugh score 7-9), and severe (Child-Pugh score 10-15) hepatic impairment. Patients with mild hepatic impairment should be given the dose of 0.5 mg/day. AZILECT should not be used in patients with moderate or severe hepatic impairment. (See CLINICAL PHARMACOLOGY, Special Populations).
PRECAUTIONS
General
Melanoma
Comparison of the rates of melanoma in the AZILECT development program with rates in age- and sex-matched populations from two epidemiologic data bases (Surveillance, Epidemiology, and End Results Registry of the National Cancer Institute and the American Academy of Dermatology Skin Cancer Screening Program) showed a risk of melanoma that was greater in patients treated with rasagiline than in the general population. Some epidemiological studies, however, have shown that patients with Parkinson’s disease have a higher risk (perhaps 2- to 4-fold higher) of developing melanoma than the general population, although it was unclear whether the observed increased risk was due to Parkinson’s disease itself or to drugs used to treat Parkinson’s disease. The increased incidence of melanoma in the AZILECT development program was comparable to the increased risk observed in the Parkinson’s disease populations examined in these epidemiological studies.
For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g., dermatologists).
Dyskinesia Due to Levodopa Treatment
When used as an adjunct to levodopa, AZILECT may potentiate dopaminergic side effects and exacerbate pre-existing dyskinesia (treatment-emergent dyskinesia occurred in about 18% of patients treated with 0.5 mg or 1 mg rasagiline as an adjunct to levodopa, and 10% of patients who received placebo as an adjunct to levodopa). Decreasing the dose of levodopa may ameliorate this side effect.
Postural Hypotension
When used as monotherapy, postural hypotension was reported in approximately 3% of patients treated with 1 mg rasagiline and 5% of patients treated with placebo. In the monotherapy trial, postural hypotension did not lead to drug discontinuation and premature withdrawal in the rasagiline or placebo-treated patients.
When used as an adjunct to levodopa, postural hypotension was reported in approximately 6% of patients treated with 0.5 mg rasagiline, 9% of patients treated with 1 mg rasagiline and 3% of patients treated with placebo. Postural hypotension led to drug discontinuation and premature withdrawal from clinical trials in one (0.7%) patient treated with rasagiline 1 mg/day, no patients treated with rasagiline 0.5 mg/day and no placebo-treated patients.
Clinical trial data suggest that postural hypotension occurs most frequently in the first two months of rasagiline treatment and tends to decrease over time.
Hallucinations
In the monotherapy study, hallucinations were reported as an adverse event in 1.3% of patients treated with 1 mg rasagiline and in 0.7% of patients treated with placebo. In the monotherapy trial, hallucinations led to drug discontinuation and premature withdrawal from clinical trials in 1.3% of the 1 mg rasagiline-treated patients and in none of the placebo-treated patients.
When used as an adjunct to levodopa, hallucinations were reported as an adverse event in approximately 5% of patients treated with 0.5 mg/day, 4% of patients treated with 1 mg/day rasagiline and 3% of patients treated with placebo. Hallucinations led to drug discontinuation and premature withdrawal from clinical trials in about 1% of patients treated with 0.5 mg/day or 1 mg/day and none of the placebo-treated patients.
Patients should be cautioned of the possibility of developing hallucinations and instructed to report them to their health care provider promptly should they develop.
Information for Patients
Patients and caregivers should be informed about which foods and beverages to avoid because of high tyramine content. They should be informed that a hypertensive crisis could occur after ingestion of certain foods (e.g., aged cheeses, pickled herring, yeast extract) or beverages (e.g., some red wines and certain beers) containing significant amounts of tyramine, or amines contained in some medications including some over-the-counter cough/cold medications. Foods high in tyramine content include those that have undergone protein change by aging, fermentation, pickling, or smoking to improve flavor such as aged cheeses, air-dried meats, sauerkraut, soy sauce, tap/draft beers and red wines. The tyramine content of any protein-rich food may be increased if stored for long periods or improperly refrigerated.
Patients and caregivers should be informed of the signs and symptoms associated with hypertensive crisis, including severe headache, blurred vision, difficulty thinking, seizures, chest pain, unexplained nausea or vomiting, or signs or symptoms of a stroke. Patients and caregivers should seek immediate medical attention for patients who develop any severe headache or other atypical or unusual symptoms not previously experienced. (See WARNINGS).
Patients should inform their physician if they are taking, or planning to take, any prescription or over-the-counter drugs, especially antidepressants and over-the-counter cold medications, since there is a potential for interaction with AZILECT. Patients should not use meperidine with AZILECT.
Patients taking AZILECT as adjunct to levodopa should be advised there is the possibility of increased dyskinesia and postural hypotension.
Patients are advised to monitor for melanomas frequently and on a regular basis. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g., dermatologists).
Patients should be instructed to take AZILECT as prescribed. If a dose is missed, the patient should not double-up the dose of AZILECT. The next dose should be taken at the usual time on the following day.
Drug Interactions
Meperidine
Serious, sometimes fatal reactions have been precipitated with concomitant use of meperidine (e.g., Demerol and other tradenames) and MAO inhibitors including selective MAO-B inhibitors. (See CONTRAINDICATIONS).
Dextromethorphan
The concomitant use of AZILECT and dextromethorphan was not allowed in clinical studies. The combination of MAO inhibitors and dextromethorphan has been reported to cause brief episodes of psychosis or bizarre behavior. Therefore, in view of AZILECT’s MAO inhibitory activity, dextromethorphan should not be used concomitantly with AZILECT. (See CONTRAINDICATIONS).
Sympathomimetic Medications
The concomitant use of AZILECT and sympathomimetic medications was not allowed in clinical studies. Severe hypertensive reactions have followed the administration of sympathomimetics and non-selective MAO inhibitors. One case of hypertensive crisis has been reported in a patient taking the recommended doses of a selective MAO-B inhibitor and a sympathomimetic medication (ephedrine). Therefore, in view of AZILECT’s MAO inhibitory activity, AZILECT should not be used concomitantly with sympathomimetics including nasal and oral decongestants and cold remedies. (See CONTRAINDICATIONS and WARNINGS, Need for Restriction of Dietary Tyramine and Amines Contained in Medications).
MAO Inhibitors
AZILECT should not be administered along with other MAO inhibitors because of the increased risk of non-selective MAO inhibition that may lead to a hypertensive crisis. (See CONTRAINDICATIONS).
Selective Serotonin Reuptake Inhibitors (SSRIs), Tricyclic and Tetracyclic Antidepressants
Concomitant use of SSRI, tricyclic, and tetracyclic antidepressants with AZILECT is not recommended (See WARNINGS).
Levodopa/carbidopa
(See CLINICAL PHARMACOLOGY, Drug-Drug Interactions; PRECAUTIONS, General, Dyskinesias Due to Levodopa Treatment).
Ciprofloxacin and Other CYP1A2 Inhibitors
Rasagiline plasma concentrations may increase up to 2 fold in patients using concomitant ciprofloxacin and other CYP1A2 inhibitors. This could result in increased adverse events. (See CLINICAL PHARMACOLOGY, Drug-Drug Interactions and WARNINGS, Ciprofloxacin and Other CYP1A2 Inhibitors).
Theophylline
(See CLINICAL PHARMACOLOGY, Drug-Drug Interactions).
Laboratory Tests
No specific laboratory tests are required for the treatment of patients on AZILECT.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Two year carcinogenicity studies were conducted in CD-1 mice at oral (gavage) doses of 1, 15, and 45 mg/kg and in Sprague-Dawley rats at oral (gavage) doses of 0.3, 1, and 3 mg/kg (males) or 0.5, 2, 5, and 17 mg/kg (females). In rats, there was no increase in tumors at any dose tested. Plasma exposures at the highest dose tested were approximately 33 and 260 times, in male and female rats, respectively, the expected plasma exposures in humans at the maximum recommended dose (MRD) of 1 mg/day.
In mice, there was an increase in lung tumors (combined adenomas/carcinomas) at 15 and 45 mg/kg males and females. Plasma exposures associated with the no-effect dose (1 mg/kg) were approximately 5 times those expected in humans at the MRD.
The carcinogenic potential of rasagiline administered in combination with levodopa/carbidopa has not been examined.
Mutagenesis
Rasagiline was reproducibly clastogenic in in vitro chromosomal aberration assays in human lymphocytes in the presence of metabolic activation and was mutagenic and clastogenic in the in vitro mouse lymphoma tk assay in the absence and presence of metabolic activation. Rasagiline was negative in the in vitro bacterial reverse mutation (Ames) assay, the in vivo unscheduled DNA synthesis assay, and the in vivo micronucleus assay in CD-1 mice. Rasagiline was also negative in the in vivo micronucleus assay in CD-1 mice when administered in combination with levodopa/carbidopa.
Impairment of Fertility
Rasagiline had no effect on mating performance or fertility in male rats treated prior to and throughout the mating period, or in female rats treated from prior to mating through day 17 of gestation at oral doses up to 3 mg/kg/day (approximately 30 times the expected plasma rasagiline exposure (AUC) at the maximum recommended human dose [1 mg/day]). The effect of rasagiline administered in combination with levodopa/carbidopa on mating and fertility has not been examined.
Pregnancy Category C
No effect on embryo-fetal development was observed in a combined mating/fertility and embryo-fetal development study in female rats at doses up to 3 mg/kg/day (approximately 30 times the expected plasma rasagiline exposure (AUC) at the maximum recommended human dose [MRHD, 1 mg/day]). Effects on embryo-fetal development in rabbit have not been adequately assessed.
In a study in which pregnant rats were dosed with rasagiline (0.1, 0.3, 1 mg/kg/day) orally, from the beginning of organogenesis to day 20 post-partum, offspring survival was decreased and offspring body weight was reduced at doses of 0.3 mg/kg/day and 1 mg/kg/day (10 and 16 times the expected plasma rasagiline exposure [AUC] at the MRHD). No plasma data were available at the no-effect dose (0.1 mg/kg); however, that dose is 1 times the MRHD on a mg/m2 basis. Rasagiline’s effect on physical and behavioral development was not adequately assessed in this study.
Rasagiline may be given as an adjunct therapy to levodopa/carbidopa treatment. In a study in which pregnant rats were dosed with rasagiline (0.1, 0.3, 1 mg/kg/day) and levodopa/carbidopa (80/20 mg/kg/day) (alone and in combination) throughout the period of organogenesis, there was an increased incidence of wavy ribs in fetuses from rats treated with rasagiline in combination with levodopa/carbidopa at 1/80/20 mg/kg/day (approximately 8 times the plasma AUC expected in humans at the MRHD and 1/1 times the MRHD of levodopa/carbidopa [800/200 mg/day] on a mg/m2 basis). In a study in which pregnant rabbits were dosed throughout the period of organogenesis with rasagiline alone (3 mg/kg) or in combination with levodopa/carbidopa (rasagiline: 0.1, 0.6, 1.2 mg/kg, levodopa/carbidopa: 80/20 mg/kg/day), an increase in embryo-fetal death was noted at rasagiline doses of 0.6 and 1.2 mg/kg/day when administered in combination with levodopa/carbidopa (approximately 7 and 13 times, respectively, the plasma rasagiline AUC at the MRHD). There was an increase in cardiovascular abnormalities with levodopa/carbidopa alone (1/1 times the MRHD on a mg/m2 basis) and to a greater extent when rasagiline (at all doses; 1-13 times the plasma rasagiline AUC at the MRHD) was administered in combination with levodopa/carbidopa.
There are no adequate and well-controlled studies of rasagiline in pregnant women. Therefore, AZILECT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
In rats rasagiline was shown to inhibit prolactin secretion and it may inhibit milk secretion in females.
It is not known whether rasagiline is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when AZILECT is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of AZILECT in the pediatric population have not been studied.
Geriatric Use
Approximately half of patients in clinical trials were 65 years and over. There were no significant differences in the safety profile of the geriatric and non-geriatric patients.
ADVERSE REACTIONS
During the clinical development of AZILECT, 1361 Parkinson’s disease patients received rasagiline as initial monotherapy or as adjunct therapy to levodopa. As these two populations differ, not only in the adjunct use of levodopa during rasagiline treatment, but also in the severity and duration of their disease, they may have differential risks for various adverse events. Therefore, most of the adverse events data in this section are presented separately for each population.
Patients receiving AZILECT as initial monotherapy treatment
Adverse Events Leading to Discontinuation in Controlled Clinical Studies:
In the double-blind, placebo-controlled trials conducted in patients receiving AZILECT as monotherapy, approximately 5% of the 149 patients treated with rasagiline discontinued treatment due to adverse events compared to 2% of the 151 patients who received placebo.
The only adverse event that led to the discontinuation of more than one patient was hallucinations.
Adverse Event Incidence in Controlled Clinical Studies:
The most commonly observed adverse events that occurred in ≥ 5% of patients receiving AZILECT 1 mg as monotherapy (n=149) participating in the double-blind, placebo-controlled trial and that were at least 1.5 times the incidence in the placebo group (n=151), were flu syndrome, arthralgia, depression, dyspepsia, and fall.
Table 6 lists treatment-emergent adverse events that occurred in ≥ 2% of patients receiving AZILECT as monotherapy participating in the double-blind, placebo-controlled trial and were numerically more frequent than in the placebo group.
| Placebo-Controlled Studies | AZILECT 1 mg | Placebo |
| Without Levodopa Treatment | (N=149) | (N=151) |
| % of Patients | % of Patients | |
| *Incidence ≥ 2% in AZILECT 1 mg group and numerically more frequent than in placebo group | ||
| Headache | 14 | 12 |
| Arthralgia | 7 | 4 |
| Dyspepsia | 7 | 4 |
| Depression | 5 | 2 |
| Fall | 5 | 3 |
| Flu syndrome | 5 | 1 |
| Conjunctivitis | 3 | 1 |
| Fever | 3 | 1 |
| Gastroenteritis | 3 | 1 |
| Rhinitis | 3 | 1 |
| Arthritis | 2 | 1 |
| Ecchymosis | 2 | 0 |
| Malaise | 2 | 0 |
| Neck Pain | 2 | 0 |
| Paresthesia | 2 | 1 |
| Vertigo | 2 | 1 |
Other events of potential clinical importance reported by 1% or more of patients receiving AZILECT as monotherapy, and at least as frequent as in the placebo group, in descending order of frequency include: dizziness, diarrhea, chest pain, albuminuria, allergic reaction, alopecia, angina pectoris, anorexia, asthma, hallucinations, impotence, leukopenia, libido decreased, liver function tests abnormal, skin carcinoma, syncope, vesiculobullous rash, vomiting.
There were no significant differences in the safety profile based on age or gender.
Patients receiving AZILECT as adjunct to levodopa therapy
Adverse Events Leading to Discontinuation in Controlled Clinical Studies:
In a double-blind, placebo-controlled trial (Study 1) conducted in patients treated with AZILECT as adjunct to levodopa therapy, approximately 9% of the 164 patients treated with AZILECT 0.5 mg/day and 7% of the 149 patients treated with AZILECT 1 mg/day discontinued treatment due to adverse events compared to 6% of the 159 patients who received placebo. The AEs that led to discontinuation of more than one rasagiline-treated patient were: diarrhea, weight loss, hallucination, and rash. Adverse event reporting was considered more reliable for Study 1 than for the second controlled trial (Study 2); therefore only the adverse event data from Study 1 are presented in this section of labeling.
Adverse Eevent Incidence in Controlled Clinical Studies:
The most commonly observed adverse events that occurred in ≥ 5% of patients receiving AZILECT 1 mg (n=149) as adjunct to levodopa therapy participating in the double-blind, placebo-controlled trial (Study 1) and that were at least 1.5 times the incidence in the placebo group (n=159) in descending order of difference in incidence were dyskinesia, accidental injury, weight loss, postural hypotension, vomiting, anorexia, arthralgia, abdominal pain, nausea, constipation, dry mouth, rash, ecchymosis, somnolence and paresthesia.
Table 7 lists treatment-emergent adverse events that occurred in ≥ 2% of patients treated with AZILECT 1 mg/day as adjunct to levodopa therapy participating in the double-blind, placebo-controlled trial (Study 1) and that were numerically more frequent than the placebo group. The table also shows the rates for the 0.5 mg group in Study 1.
| AZILECT | AZILECT | ||
| 1 mg + | 0.5 mg + | Placebo + | |
| Levodopa | Levodopa | Levodopa | |
| (N=149) | (N=164) | (N=159) | |
| % of patients | % of patients | % of patients | |
| *Incidence ≥ 2% in AZILECT 1 mg group and numerically more frequent than in placebo group | |||
| Dyskinesia | 18 | 18 | 10 |
| Accidental injury | 12 | 8 | 5 |
| Nausea | 12 | 10 | 8 |
| Headache | 11 | 8 | 10 |
| Fall | 11 | 12 | 8 |
| Weight loss | 9 | 2 | 3 |
| Constipation | 9 | 4 | 5 |
| Postural hypotension | 9 | 6 | 3 |
| Arthralgia | 8 | 6 | 4 |
| Vomiting | 7 | 4 | 1 |
| Dry mouth | 6 | 2 | 3 |
| Rash | 6 | 3 | 3 |
| Somnolence | 6 | 4 | 4 |
| Abdominal pain | 5 | 2 | 1 |
| Anorexia | 5 | 2 | 1 |
| Diarrhea | 5 | 7 | 4 |
| Ecchymosis | 5 | 2 | 3 |
| Dyspepsia | 5 | 4 | 4 |
| Paresthesia | 5 | 2 | 3 |
| Abnormal dreams | 4 | 1 | 1 |
| Hallucinations | 4 | 5 | 3 |
| Ataxia | 3 | 6 | 1 |
| Dyspnea | 3 | 5 | 2 |
| Infection | 3 | 2 | 2 |
| Neck pain | 3 | 1 | 1 |
| Sweating | 3 | 2 | 1 |
| Tenosynovitis | 3 | 1 | 0 |
| Dystonia | 3 | 2 | 1 |
| Gingivitis | 2 | 1 | 1 |
| Hemorrhage | 2 | 1 | 1 |
| Hernia | 2 | 1 | 1 |
| Myasthenia | 2 | 2 | 1 |
Several of the more common adverse events seemed dose-related, including weight loss, postural hypotension, and dry mouth.
Other events of potential clinical importance reported in Study 1 by 1% or more of patients treated with rasagiline 1 mg/day as adjunct to levodopa therapy, and at least as frequent as in the placebo group, in descending order of frequency include: skin carcinoma, anemia, albuminuria, amnesia, arthritis, bursitis, cerebrovascular accident, confusion, dysphagia, epistaxis, leg cramps, pruritus, skin ulcer.
There were no significant differences in the safety profile based on age or gender.
Other Adverse Events Observed During All Phase 2/3 Clinical Trials
Rasagiline was administered to approximately 1361 patients during all PD phase 2/3 clinical trials. About 283 patients received rasagiline for at least one year, approximately 410 patients received rasagiline for at least two years, 116 patients received rasagiline for at least 3 years, and 245 patients received rasagiline for more than 3 years, with some patients treated for more than 5 years. The long-term safety profile was similar to that observed with shorter duration exposure.
The frequencies listed below represent the proportion of the 1361 individuals exposed to rasagiline who experienced events of the type cited.
All events that occurred at least twice (or once for serious or potentially serious events), except those already listed above, trivial events, terms too vague to be meaningful, adverse events with no plausible relation to treatment, and events that would be expected in patients of the age studied were reported without regard to determination of a causal relationship to rasagiline.
Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in at least 1/100 patients, infrequent adverse events are defined as those occurring in at least 1/100 to 1/1000 patients and rare adverse events are defined as those occurring in fewer than 1/1000 patients.
Body as a whole: Frequent: asthenia Infrequent: chills, face edema, flank pain, photosensitivity reaction
Cardiovascular system: Frequent: bundle branch block Infrequent: deep thrombophlebitis, heart failure, migraine, myocardial infarct, phlebitis, ventricular tachycardia Rare: arterial thrombosis, atrial arrhythmia, AV block complete, AV block second degree, bigeminy, cerebral hemorrhage, cerebral ischemia, ventricular fibrillation
Digestive system: Frequent: gastrointestinal hemorrhage Infrequent: colitis, esophageal ulcer, esophagitis, fecal incontinence, intestinal obstruction, mouth ulceration, stomach ulcer, stomatitis, tongue edema Rare: hematemesis, hemorrhagic gastritis, intestinal perforation, intestinal stenosis, jaundice, large intestine perforation, megacolon, melena
Hemic and Lymphatic system: Infrequent: macrocytic anemia Rare: purpura, thrombocythemia
Metabolic and Nutritional disorders: Infrequent: hypocalcemia
Musculoskeletal system: Infrequent: bone necrosis, muscle atrophy Rare: arthrosis
Nervous system: Frequent: abnormal gait, anxiety, hyperkinesia, hypertonia, neuropathy, tremor Infrequent: agitation, aphasia, circumoral paresthesia, convulsion, delusions, dementia, dysarthria, dysautonomia, dysesthesia, emotional lability, facial paralysis, foot drop, hemiplegia, hypesthesia, incoordination, manic reaction, myoclonus, neuritis, neurosis, paranoid reaction, personality disorder, psychosis, wrist drop Rare: apathy, delirium, hostility, manic depressive reaction, myelitis, neuralgia, psychotic depression, stupor
Respiratory system: Frequent: cough increased Infrequent: apnea, emphysema, laryngismus, pleural effusion, pneumothorax Rare: interstitial pneumonia, larynx edema, lung fibrosis
Skin and Appendages: Infrequent: eczema, urticaria Rare: exfoliative dermatitis, leukoderma
Special senses: Infrequent: blepharitis, deafness, diplopia, eye hemorrhage, eye pain, glaucoma, keratitis, ptosis, retinal degeneration, taste perversion, visual field defect Rare: blindness, parosmia, photophobia, retinal detachment, retinal hemorrhage, strabismus, taste loss, vestibular disorder
Urogenital system: Frequent: hematuria, urinary incontinence Infrequent: acute kidney failure, dysmenorrhea, dysuria, kidney calculus, nocturia, polyuria, scrotal edema, sexual function abnormal, urinary retention, urination impaired, vaginal hemorrhage, vaginal moniliasis, vaginitis Rare: abnormal ejaculation, amenorrhea, anuria, epididymitis, gynecomastia, hydroureter, leukorrhea, priapism
DRUG ABUSE AND DEPENDENCE
AZILECT is not a controlled substance.
Studies conducted in mice and rats did not reveal any potential for drug abuse and dependence. Clinical trials have not revealed any evidence of the potential for abuse, tolerance or physical dependence; however, systematic studies in humans designed to evaluate these effects have not been performed.
OVERDOSE
No cases of AZILECT overdose were reported in clinical trials.
Rasagiline was well tolerated in a single-dose study in healthy volunteers receiving 20 mg/day and in a ten-day study in healthy volunteers receiving 10 mg/day. Adverse events were mild or moderate. In a dose escalation study in patients on chronic levodopa therapy treated with 10 mg of rasagiline there were three reports of cardiovascular side effects (including hypertension and postural hypotension) which resolved following treatment discontinuation.
Symptoms of overdosage, although not observed with rasagiline during clinical development, may resemble those observed with non-selective MAO inhibitors.
Although no cases of overdose have been observed with rasagiline, the following description of presenting symptoms and clinical course is based upon overdose descriptions of non-selective MAO inhibitors.
Characteristically, signs and symptoms of non-selective MAOI overdose may not appear immediately. Delays of up to 12 hours between ingestion of drug and the appearance of signs may occur. Importantly, the peak intensity of the syndrome may not be reached for upwards of a day following the overdose. Death has been reported following overdosage. Therefore, immediate hospitalization, with continuous patient observation and monitoring for a period of at least two days following the ingestion of such drugs in overdose, is strongly recommended.
The clinical picture of MAOI overdose varies considerably; its severity may be a function of the amount of drug consumed. The central nervous and cardiovascular systems are prominently involved.
Signs and symptoms of overdosage may include, alone or in combination, any of the following: drowsiness, dizziness, faintness, irritability, hyperactivity, agitation, severe headache, hallucinations, trismus, opisthotonos, convulsions, and coma; rapid and irregular pulse, hypertension, hypotension and vascular collapse; precordial pain, respiratory depression and failure, hyperpyrexia, diaphoresis, and cool, clammy skin.
There is no specific antidote for rasagiline overdose. The following suggestions are offered based upon the assumption that rasagiline overdose may be modeled after non-selective MAO inhibitor poisoning. Treatment of overdose with non-selective MAO inhibitors is symptomatic and supportive. Respiration should be supported by appropriate measures, including management of the airway, use of supplemental oxygen, and mechanical ventilatory assistance, as required. Body temperature should be monitored closely. Intensive management of hyperpyrexia may be required. Maintenance of fluid and electrolyte balance is essential.
A poison control center should be called for the most current treatment guidelines.
DOSAGE AND ADMINISTRATION
Tyramine-rich foods, beverages, or dietary supplements and amines (from over-the-counter cough/cold medications) should be avoided to prevent a possible hypertensive crisis/“cheese reaction” during rasagiline treatment. (See WARNINGS, Need for Restriction of Dietary Tyramine and Amines Contained in Medications).
Monotherapy
The recommended AZILECT dose for the treatment of Parkinson’s disease patients is 1 mg administered once daily.
Adjunctive Therapy
The recommended initial dose is 0.5 mg administered once daily. If a sufficient clinical response is not achieved, the dose may be increased to 1 mg administered once daily.
Change of Levodopa Dose in Adjunct Therapy
When AZILECT is used in combination with levodopa, a reduction of the levodopa dosage may be considered based upon individual response. During the controlled trials of AZILECT as adjunct therapy to levodopa, levodopa dosage was reduced in some patients. In clinical studies, dosage reduction of levodopa was allowed within the first 6 weeks if dopaminergic side effects, including dyskinesia and hallucinations, emerged. In Study 1, levodopa dosage reduction occurred in 8% of patients in the placebo group and in 16% and 17% of patients in the 0.5 mg/day and 1 mg/day rasagiline groups, respectively. In those patients who had levodopa dosage reduced, the dose was reduced on average by about 7%, 9%, and 13% in the placebo, 0.5 mg/day, and 1 mg/day groups, respectively. In Study 2, levodopa dosage reduction occurred in 6% of patients in the placebo group and in 9% in the rasagiline 1 mg/day group. In patients who had their levodopa dosage reduced, the dose was reduced on average by about 13% and 11% in the placebo and the rasagiline groups, respectively.
Patients with Hepatic Impairment
AZILECT plasma concentrations will increase in patients with hepatic impairment. Patients with mild hepatic impairment should use 0.5 mg daily of AZILECT. AZILECT should not be used in patients with moderate or severe hepatic impairment (See CLINICAL PHARMACOLOGY, Special Populations, Hepatic Insufficiency and WARNINGS, Hepatic Insufficiency).
Patients Taking Ciprofloxacin and Other CYP1A2 Inhibitors
Rasagiline plasma concentrations are expected to double in patients taking concomitant ciprofloxacin and other CYP1A2 inhibitors. Therefore, patients taking concomitant ciprofloxacin or other CYP1A2 inhibitors should use 0.5 mg daily of AZILECT. (See CLINICAL PHARMACOLOGY, Drug-Drug Interactions, Ciprofloxacin and Effect of other drugs on the metabolism of AZILECT; and WARNINGS, Ciprofloxacin and Other CYP1A2 Inhibitors).
HOW SUPPLIED
AZILECT 0.5 mg Tablets:
White to off-white, round, flat, beveled tablets, debossed with “GIL 0.5” on one side and plain on the other side. Supplied as bottles of 30 tablets (NDC 68546-142-56).
AZILECT 1 mg Tablets:
White to off-white, round, flat, beveled tablets, debossed with “GIL 1” on one side and plain on the other side. Supplied as bottles of 30 tablets (NDC 68546-229-56).
Storage: Store at 25°C (77°F) with excursions permitted to 15°-30°C (59°-86°F).
Rx only
Manufactured by:
Teva Pharmaceutical Industries Ltd.
Kfar Saba 44102, Israel
Marketed by:
Teva Neuroscience, Inc.
Kansas City, MO 64131
Revision 05/06
| AZILECT (rasagiline mesylate) | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| AZILECT (rasagiline mesylate) | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
Revised: 06/2006