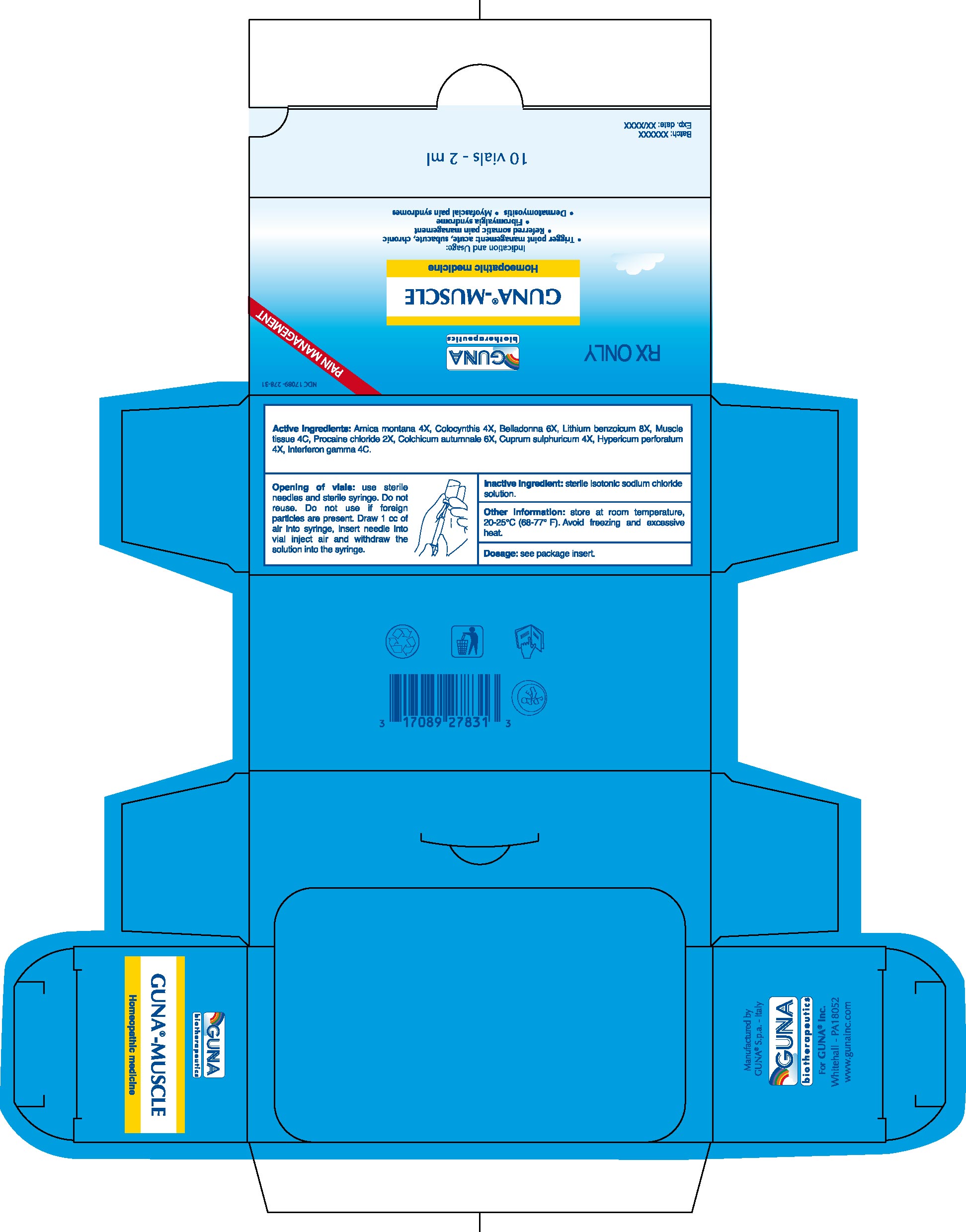
GUNA-MUSCLE
-
arnica montana,
atropa belladonna,
colchicum autumnale bulb,
citrullus colocynthis fruit pulp,
cupric sulfate,
hypericum perforatum,
interferon gamma-1b,
lithium benzoate,
pork and
procaine hydrochloride injection, solution
Guna spa
----------
GUNA®-MUSCLE (Homeopathic complex preparation) INJECTION SC, ID, IM1. INDICATIONS AND USAGE
1.1. Trigger point management: acute, subacute, chronic
1.2. Referred somatic pain management (use with GUNA®-NEURAL)
1.3. Fibromyalgia syndrome (use with GUNA®-NEURAL)
1.4. Dermatomyositis.
1.5. Myofascial pain syndromes (use with GUNA®-NEURAL)
2. DOSAGE AND ADMINISTRATION
2.1. Standard protocol for IM administration: 1 vial 1-3 times a week according to severity and clinical progress.
2.2. Standard protocol according to mesotherapy technique using 1 vial per treatment: 2 treatments for the first 2 weeks, 1 treatment a week till pain relief (average 8-10 sessions). For chronic pathologies: continue 1 treatment a week for 1 month till pain relief, then 1 treatment a month.
Select application site according to trigger points, tender points, referred pain zones, acupuncture points, nerve key points, Head zones or “local pain points”. Using a 13 mm, 30G or a 4 mm, 27G needle, make the classic intradermal injection according to mesotherapy technique.
Discard unused solution.
2.3. Opening of Vials: Use sterile needles and sterile syringe. Do not reuse. Do not use if foreign particles are present. Draw 1 cc of air into syringe, insert needle into vial inject air and withdraw the solution.
3. DOSAGE FORMS AND STRENGTHS
3.1. 2 ml glass vials
Each ingredient is attenuated according to the procedure stated in the Homeopathic Pharmacopeia of the United States.
Active ingredients: Arnica montana 4X, Colocynthis 4X, Belladonna 6X, Lithium benzoicum 8X, Muscle tissue 4C, Procaine chloride 2X, Colchicum autumnale 6X, Cuprum sulphuricum 4X, Hypericum perforatum 4X, Interferon gamma 4C.
Inactive ingredient: Sterile isotonic sodium chloride solution
4. CONTRAINDICATIONS
4.1. In rare cases patients may experience hypersalivation within one hour of treatment. Discontinue treatment. The hypersalivation resolves over several hours without further medical treatment.
5. WARNINGS AND PRECAUTIONS
5.1. Muscle pain requires differential diagnosis for segmental nerve pain, plantar fasciitis, tendinitis, tendinosis, deep blood accumulation/hematoma.
5.2. Skin cleansing/disinfection is required before application. Saprophytic bacteria may produce injection site abscesses with improper skin preparation.
5.3. In rare cases patients may experience hypersalivation within one hour of treatment. Discontinue treatment. The hypersalivation resolves over several hours without further medical treatment.
5.4. Patients with known sensitivity to procaine or other local anesthetics should be kept under observation during the therapy.
6. ADVERSE REACTIONS
6.1 The most common mild adverse reaction is slight reddening at the injection site due to the mechanical effect of the needle or a superficial skin reaction of mild erythema.
7. DRUG INTERACTIONS
7.1. None Known.
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy: Pregnancy category C. Animal reproduction studies have not been conducted with GUNA®-MUSCLE. GUNA®- MUSCLE should not be given to a pregnant woman.
8.2 Nursing mothers: It is not known whether any of the ingredients in GUNA®-MUSCLE are secreted in human milk. However, since many drugs are secreted in human milk, caution should be exercised when GUNA®- MUSCLE is administered to a nursing woman.
8.3 Pediatric use: No restrictions.
8.4 Geriatric use: No restrictions.
9. DRUG ABUSE AND DEPENDENCE
9.1. No Known.
10. OVERDOSAGE
10.1. No Known.
11. DESCRIPTION
11.1. GUNA®-MUSCLE is a sterile solution made with isotonic sodium chloride solution.
It is a homeopathic complex medicine, whose active ingredients have been selected in order to promote 2 main activities:
• Detoxification of the connective tissue matrix
• Pain modulation through stimulation of the physiological mechanism of pain control.
Attenuation of the biological substrates acts to target the area of activity of the product.
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
Due to the homeopathic nature of the active ingredients, receptors may be activated by feedback regulation. Beta-endorphins at the 4C dose activate the membrane receptors for endogenous endorphins that play a key role in pain relief.
12.2. Pharmacodynamics
The physiological effects of GUNA®-MUSCLE are due to the action of the ingredients, as described in the Homeopathic Materia Medica.
In homeopathy there is no direct relationship between dose and effect, but rather there is a relationship between attenuation and a balancing effect on biochemical pathways.
In GUNA®-MUSCLE the attenuation of each ingredient has been selected according to the Arndt-Schulz Principle (inverted effect law). The attenuation of the physiological ingredients promotes membrane receptor feedback in order to normalize altered biological pathways. In Addition the attenuation technique activates the low dilutions and stabilizes clinical activity of the compound.
12.3. Pharmacokinetics
Homeopathic attenuation provides complete bioavailability of the active ingredients.
13. NONCLINICAL TOXICOLOGY
13.1. GUNA®-MUSCLE has no level of toxicity due to the attenuation of the ingredients.
14. CLINICAL STUDIES
14.1 GUNA®-MUSCLE formulation is based on classical Homeopathy and each ingredient has been selected according to its description in the Homeopathic Materia Medica. The product is intended for application to target points such as acupuncture points, Weihe points, and key neurological points.
15. REFERENCES
15.1. I. Bianchi: Citochine e Interferoni. Farmacologia e Clinica. Nuova IPSA Editore.
15.2. L. Milani: Weihe e altri Punti tra Agopuntura e Omeopatia. Guna Editore.
15.3. J. Malzac: Materia Medica Immunologia. IPSA Editore.
15.4. H.H. Reckeweg: Homeopathic Materia Medica. Aurelia Verlag.
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1. NDC 17089-278-31 10 glass vials packaged in carton box16.2. NDC 17089-278-32 50 glass vials packaged in carton box
16.3. Store at room temperature, 20-25°C (68-77° F). Avoid freezing and excessive heat.
17. PATIENT COUNSELING INFORMATION
17.1. Patients should be informed about Homeopathy and Acupuncture and the main differences from the conventional clinical approach.
PACKAGE LABEL
| GUNA-MUSCLE
arnica montana - atropa belladonna - colchicum autumnale bulb - cupric sulfate - hypericum perforatum - interferon gamma-1b - lithium benzoate - pork - procaine hydrochloride - citrullus colocynthis fruit pulp - injection, solution |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved homeopathic | 09/29/2006 | ||
| Labeler - Guna spa (430538264) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Guna spa | 430538264 | manufacture | |