ZICAM
-
zinc acetate and
zinc gluconate tablet, orally disintegrating
Matrixx Initiatives, Inc.
----------
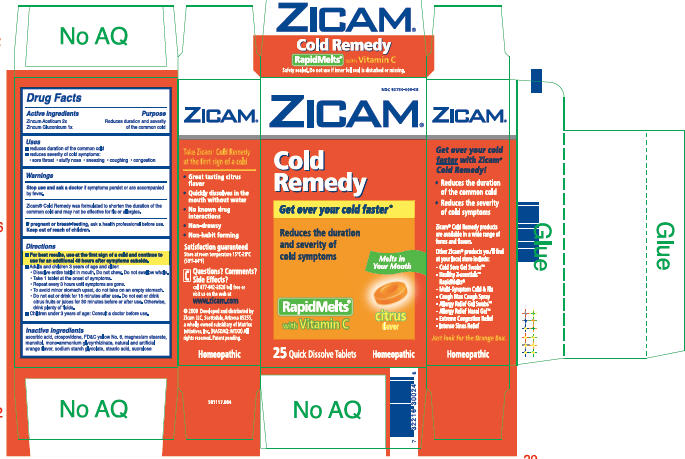
ZICAM®Cold Remedy
RapidMelts®
Drug Facts
| Active ingredients | Purpose |
|---|---|
| Zincum Aceticum 2x | Reduces duration and severity |
| Zincum Gluconicum 1x | of the common cold |
Uses
- reduces duration of the common cold
- reduces severity of cold symptoms:
- sore throat
- stuffy nose
- sneezing
- coughing
- congestion
Warnings
Stop use and ask a doctor if symptoms persist or are accompanied by fever.
Zicam® Cold Remedy was formulated to shorten the duration of the common cold and may not be effective for flu or allergies.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
Directions
- For best results, use at the first sign of a cold and continue to use for an additional 48 hours after symptoms subside.
- Adults and children 3 years of age and older:
- Dissolve entire tablet in mouth. Do not chew. Do not swallow whole.
- Take 1 tablet at the onset of symptoms.
- Repeat every 3 hours until symptoms are gone.
- To avoid minor stomach upset, do not take on an empty stomach.
- Do not eat or drink for 15 minutes after use. Do not eat or drink citrus fruits or juices for 30 minutes before or after use. Otherwise, drink plenty of fluids.
- Children under 3 years of age: Consult a doctor before use.
Inactive ingredients
ascorbic acid, crospovidone, FD&C yellow No. 6, magnesium stearate, mannitol, mono-ammonium glycyrrhizinate, natural and artificial orange flavor, sodium starch glycolate, stearic acid, sucralose
Questions? Comments? Side Effects?
call 877-942-2626 toll free or visit us on the web at
www.zicam.com
PRINCIPAL DISPLAY PANEL - 25 Tablet Carton
NDC 62750-008-28
ZICAM®
Cold
Remedy
Get over your cold faster®
Reduces the duration
and severity of
cold symptoms
Melts in
Your Mouth
RapidMelts®
with Vitamin C
citrus
flavor
25 Quick Dissolve Tablets
Homeopathic
| ZICAM
zinc acetate and zinc gluconate tablet, orally disintegrating |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved homeopathic | 07/21/2006 | ||
| Labeler - Matrixx Initiatives, Inc. (790037253) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Nutritional Labs International | 007306970 | Analysis, Manufacture, Relabel, Repack | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Merical | 808183953 | Relabel, Repack | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Boston Analytical, Inc. | 147830830 | Analysis | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| TLC Labs, Inc | 169896730 | Analysis | |