androderm (Testosterone) patch
[Watson Pharma, Inc.]
DESCRIPTION
Androderm (testosterone transdermal system) provides continuous delivery of testosterone (the primary endogenous androgen) for 24 hours following application to intact, non-scrotal skin (e.g., back, abdomen, thighs, upper arms).
Two strengths of Androderm are available which deliver in vivo 2.5 mg or 5 mg of testosterone per day across skin of average permeability.
Androderm has a central drug delivery reservoir surrounded by a peripheral adhesive area. The Androderm 2.5 mg system has a total contact surface area of 37 cm2 with a 7.5 cm2 central drug delivery reservoir containing 12.2 mg testosterone USP, dissolved in an alcohol-based gel. The Androderm 5 mg system has a total contact surface area of 44 cm2 with a 15 cm2 central drug delivery reservoir containing 24.3 mg testosterone USP, dissolved in an alcohol-based gel. Testosterone USP is a white, or creamy white crystalline powder or crystals chemically described as 17ß-hydroxyandrost-4-en-3-one.
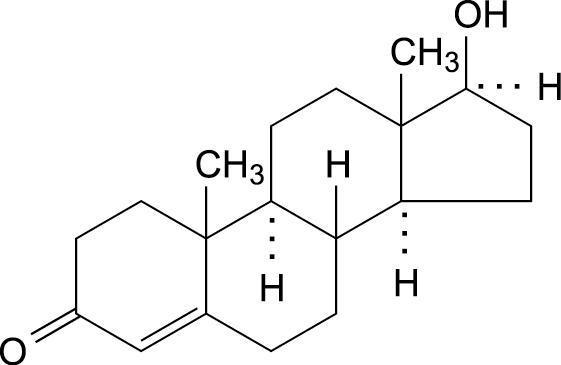
Testosterone C19H28O2 mw 288.43
The Androderm systems have six components as shown in Figure 1. Proceeding from the top toward the surface attached to the skin, the system is composed of (1) metallized polyester/Surlyn®1 (ethylene-methacrylic acid copolymer)/ethylene vinyl acetate backing film with alcohol resistant ink, (2) a drug reservoir of testosterone USP, alcohol USP, glycerin USP, glycerol monooleate, methyl laurate, sodium hydroxide NF, to adjust pH, and purified water USP, gelled with carbomer copolymer Type B NF, (3) a permeable polyethylene microporous membrane, and (4) a peripheral layer of acrylic adhesive surrounding the central, active drug delivery area of the system. Prior to opening of the system and application to the skin, the central delivery surface of the system is sealed with a peelable laminate disc (5) composed of a five-layer laminate containing polyester/polyesterurethane adhesive/aluminum foil/polyester-urethane adhesive/polyethylene. The disc is attached to and removed with the release liner (6), a silicone-coated polyester film, which is removed before the system can be used.
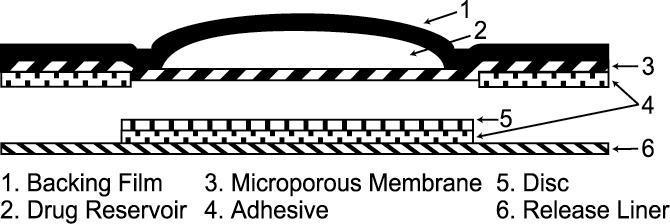
Figure 1: System Schematic
The active ingredient in the system is testosterone. The remaining components of the system are pharmacologically inactive.
- 1
- Surlyn is a registered trademark of E.I. DuPont de Nemours & Company.
CLINICAL PHARMACOLOGY
Androderm (testosterone transdermal system) delivers physiologic amounts of testosterone producing circulating testosterone concentrations that approximate the normal circadian rhythm of healthy young men.
Testosterone
Androderm (testosterone transdermal system) delivers testosterone, the primary androgenic hormone. Testosterone is responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of the prostate, seminal vesicles, penis, and scrotum; development of male hair distribution, such as facial, pubic, chest, and axillary hair; laryngeal enlargement; vocal cord thickening; and alterations in body musculature and fat distribution.
Male hypogonadism results from insufficient secretion of testosterone and is characterized by low serum testosterone concentrations. Symptoms associated with male hypogonadism include the following: impotence and decreased sexual desire; fatigue and loss of energy; mood depression; and regression of secondary sexual characteristics.
General Androgen Effects
Androgens promote retention of nitrogen, sodium, potassium, and phosphorus, and decreased urinary excretion of calcium. Androgens have been reported to increase protein anabolism and decrease protein catabolism. Nitrogen balance is improved only when there is sufficient intake of calories and protein.
Androgens are also responsible for the growth spurt of adolescence and for the eventual termination of linear growth that is brought about by the fusion of the epiphyseal growth centers. In children, exogenous androgens accelerate linear growth rates but may cause disproportionate advancement in bone maturation. Use over long periods may result in fusion of the epiphyseal growth centers and termination of the growth process.
Androgens have been reported to stimulate the production of red blood cells by enhancing erythropoietin production.
During exogenous administration of androgens, endogenous testosterone release is inhibited through feedback inhibition of pituitary LH secretion. With large doses of exogenous androgens, spermatogenesis may also be suppressed through feedback inhibition of pituitary follicle stimulating hormone (FSH) secretion.
There is a lack of substantial evidence that androgens are effective in accelerating fracture healing or in shortening post-surgical convalescence.
Pharmacokinetics
Absorption
Following Androderm (testosterone transdermal system) application to non-scrotal skin, testosterone is continuously absorbed during the 24-hour dosing period. Daily application of Androderm at approximately 10 PM results in a serum testosterone concentration profile that mimics the normal circadian variation observed in healthy young men (Fig. 2 below). Maximum concentrations occur in the early morning hours with minimum concentrations in the evening (Table 1 below).
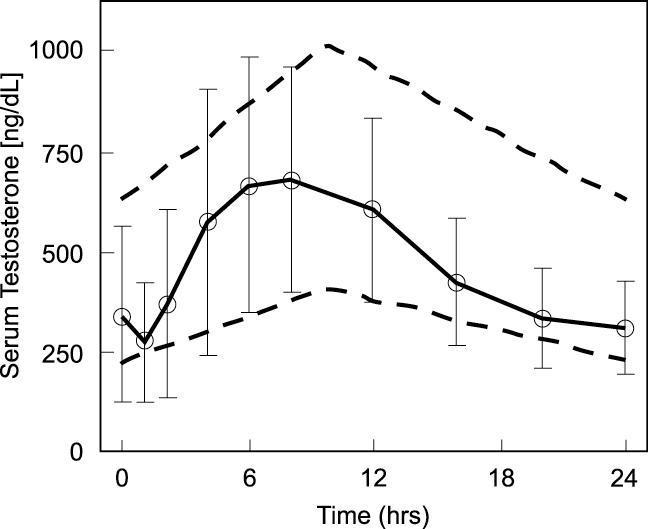
Figure 2: Mean (SD) steady state serum testosterone concentrations during nightly application of Androderm 2.5 mg systems in 29 hypogonadal male patients, 27 patients used 2 systems nightly and 2 patients used 3 systems nightly. Area between the dashed lines shows the 95% confidence interval for the circadian variation observed in healthy young men.1 System application (t=0) at approximately 10 PM.
| Parameter | Units | n | Mean | SD |
| Cmax = maximum serum concentration | ||||
| Cavg= average serum concentration (AUC/24 hr) | ||||
| Cmin = minimum serum concentration | ||||
| Tmax = time of maximum serum concentration | ||||
| T½ = elimination half-life | ||||
| CL = clearance | ||||
| Cmax | ng/dL | 56 | 753 | 276 |
| Cavg | ng/dL | 56 | 498 | 169 |
| Cmin | ng/dL | 56 | 246 | 120 |
| Tmax | hr | 56 | 7.9 | 2.2 |
| T½ | min | 29 | 71 | 32 |
| CL | L/day | 49 | 1304 | 464 |
In a group of 34 hypogonadal men, application of two Androderm 2.5 mg systems to the abdomen, back, thighs, or upper arms resulted in average testosterone absorption of 4 to 5 mg over 24 hours. The serum testosterone concentration profiles during application were similar for these sites (Table 2). Applications to the chest and shins resulted in greater inter-individual variability and average 24 hour absorption of 3 to 4 mg.
| Sample | Abdomen | Back | Thigh | Upper Arm | ||||
|---|---|---|---|---|---|---|---|---|
| Time (hr) | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 0 | 90 | 82 | 80 | 74 | 85 | 76 | 81 | 69 |
| 3 | 286 | 201 | 429 | 252 | 271 | 201 | 308 | 226 |
| 6 | 476 | 236 | 608 | 250 | 489 | 254 | 468 | 245 |
| 9 | 570 | 234 | 613 | 214 | 592 | 251 | 534 | 204 |
| 12 | 575 | 244 | 588 | 233 | 594 | 247 | 527 | 199 |
| 24 | 352 | 164 | 403 | 174 | 367 | 161 | 332 | 124 |
In a steady-state study of 12 hypogonadal men, nightly application of 1, 2, or 3 Androderm 2.5 mg systems resulted in increases in the mean morning serum testosterone concentrations. These concentrations averaged 424, 584, and 766 ng/dL with the application of 1, 2, and 3 systems, respectively. The mean baseline serum testosterone concentration was 76 ng/dL.
Normal range morning serum testosterone concentrations are reached during the first day of dosing. There is no accumulation of testosterone during continuous treatment.
In a study of 20 hypogonadal patients, two Androderm 2.5 mg systems and a single Androderm 5 mg system produced equivalent serum testosterone concentration profiles. Average steady state concentrations over 24 hours (Cssavg) were 613±169 and 621±176 ng/dL for the two 2.5 mg and single 5 mg systems, respectively. Cmax values were 925±340 ng/dL for the two 2.5 mg systems and 905±254 ng/dL for the single 5 mg system.
In 16 hypogonadal men, the topical administration of 0.1% triamcinolone cream to the skin under the central drug reservoir prior to application of the Androderm system did not significantly alter transdermal absorption of testosterone; however, the rate of complete adherence was lower. In these patients, pretreatment with an ointment formulation significantly reduced testosterone absorption from the system.
Distribution
In serum, testosterone is bound with high affinity to sex hormone binding globulin (SHBG) and with low affinity to albumin. The albumin bound portion easily dissociates and is presumed to be bioactive. The SHBG-bound portion is not considered to be bioactive. The amount of SHBG in serum and the total testosterone concentration determine the distribution of bioactive and non-bioactive androgen.
Bioactive serum testosterone concentrations (BT) measured during Androderm (testosterone transdermal system) treatment paralleled the serum testosterone profile (Figure 2) and remained within the normal reference range.
Metabolism
Inactivation of testosterone occurs primarily in the liver. Testosterone (T) is metabolized to various 17-keto steroids through two different pathways, and the major active metabolites are estradiol (E2) and dihydrotestosterone (DHT). DHT binds with greater affinity to SHBG than does testosterone. In reproductive tissues, DHT is further metabolized to 3-alpha and 3-beta androstanediol.
In many tissues, the activity of testosterone appears to depend on reduction to DHT, which binds to cytosol receptor proteins. The steroid-receptor complex is transported to the nucleus, where it initiates transcription events and cellular changes related to androgen action.
During steady-state pharmacokinetic studies in hypogonadal men treated with Androderm, the average DHT:T and E2:T ratios were comparable to those in normal men, approximately 1:10 and 1:200, respectively.
Upon removal of the Androderm systems, serum testosterone concentrations decrease with an apparent half-life of approximately 70 minutes. Hypogonadal concentrations are reached within 24 hours following system removal.
Androderm therapy suppresses endogenous testosterone secretion via the pituitary/gonadal axis, resulting in a reduction in baseline serum testosterone concentrations compared to the untreated state.
Excretion
Approximately 90% of a testosterone dose given intramuscularly is excreted in the urine as glucuronide and sulfate conjugates of testosterone and its metabolites; about 6% is excreted in the feces, mostly in unconjugated form.
Special Populations
Geriatric
No age related effects on testosterone pharmacokinetics were observed in clinical trials of Androderm in men up to 65 years of age. In a group of 9 elderly testosterone deficient men (65-79 years of age, average baseline testosterone level 184±50 ng/dL), a single application of two Androderm 2.5 mg systems to the back resulted in an average testosterone level of 591±121 ng/dL with a Tmax of 14.2±4.2 hours. The total testosterone delivered over the 24-hour application time was 3.8±0.6 mg, approximately 20% less than the average amount delivered in younger patients.
Race
There is insufficient information available from Androderm trials to compare testosterone pharmacokinetics in different racial groups.
Renal Insufficiency
There is no experience with use of Androderm in patients with renal insufficiency.
Hepatic Insufficiency
There is no experience with use of Androderm in patients with hepatic insufficiency.
Drug-Drug Interactions
See “ Precautions” below
Clinical Studies
In clinical studies using the Androderm 2.5 mg system, 93% of patients were treated with two systems daily, 6% used three systems daily, and 1% used one system daily.
The hormonal effects of Androderm (testosterone transdermal system) as a treatment for male hypogonadism were demonstrated in four open-label trials that included 94 hypogonadal men, ages 15 to 65 years. In these trials, Androderm produced average morning serum testosterone concentrations within the normal reference range in 92% of patients. The mean (SD) serum hormone concentrations and percentage of patients who achieved average concentrations within the normal ranges are shown in Table 3 below.
| T | BT | DHT | E2 | |
| Normal Range | (306-1031) | (93-420) | (28-85) | (0.9-3.6) |
| Mean | 589 | 312 | 47 | 2.7 |
| SD | 209 | 127 | 18 | 1.2 |
| % Normal | 92 | 88 | 85 | 77 |
| % High | 1 | 12 | 2 | 22 |
| % Low | 7 | 0 | 13 | 1 |
A physiological suppression of the pituitary/gonadal axis occurs during continuous Androderm treatment leading to reduced serum LH concentrations. In clinical trials, 10 of 21 (48%) of men with primary (hypergonadotropic) hypogonadism achieved normal range LH concentrations within 6 to 12 months of treatment. LH concentrations may remain elevated in some patients despite serum testosterone concentrations within the normal range.
Twenty-nine patients, previously treated with testosterone, completed 12 months of Androderm treatment. Following an 8-week androgen withdrawal period, Androderm treatment produced positive effects on fatigue, mood and sexual function. The percent of patients complaining of fatigue decreased from 79% to 10% during treatment (p<0.001). The average patient depression score (Beck Depression Inventory) decreased from 6.9 to 3.9 (p<0.001). Nocturnal penile tumescence and rigidity monitoring showed an increase in mean duration of erections 0.23 to 0.39 hours per night (p=0.01) and an increase in penile tip rigidity from 18% to 50% (p<0.001). The total number of self-reported erections reported increased from 2.3 to 7.8 per week (p<0.001).
Comparison with intramuscular testosterone: Sixty-six patients, previously treated with testosterone injections, received Androderm or intramuscular testosterone enanthate (200 mg every 2 weeks) treatment for 6 months. The percent of time that serum concentrations measured throughout the dosing interval remained within the normal range were as follows:
| Androderm | IM | p value | |
| Sexual function was comparable between groups | |||
| T | 82% | 72% | 0.05 |
| BT | 87% | 39% | <0.001 |
| DHT | 76% | 70% | 0.06 |
| E2 | 81% | 35% | <0.001 |
Effect on plasma lipids: In 67 men treated for 6 to 12 months, the average (SE) serum total cholesterol and HDL concentrations were 199 (7.6) ng/dL and 46 (2.3) ng/dL.
Compared to baseline values during a hypogonadal state achieved by 8 weeks of androgen withdrawal in 29 patients, the following changes in lipids were observed during 1 year of Androderm treatment: Cholesterol decreased 1.2%; HDL decreased 8%; Cholesterol/HDL ratio increased 9%. In these patients, lipids measured during Androderm treatment were not significantly different from those measured during prior IM injection treatment.
Effects on the prostate: Prostate size and serum prostate specific antigen (PSA) concentrations during treatment were comparable to values reported for eugonadal men. One case of prostate carcinoma occurred during Androderm treatment; two cases were detected during IM treatment.
INDICATIONS AND USAGE
Androderm (testosterone transdermal system) is indicated for testosterone replacement therapy in men for conditions associated with a deficiency or absence of endogenous testosterone.
Primary hypogonadism (congenital or acquired)—Testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, or orchidectomy, Klinefelter’s syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone concentrations accompanied by gonadotropins (FSH, LH) above the normal range.
Secondary, i.e., hypogonadotropic hypogonadism (congenital or acquired)—idiopathic gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency, or pituitary-hypothalamic injury from tumors, trauma, or radiation. These men have low serum testosterone concentrations without associated elevation in gonadotropins. Appropriate adrenal cortical and thyroid hormone replacement therapy may be necessary in patients with multiple pituitary or hypothalamic abnormalities.
CONTRAINDICATIONS
Androgens are contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate.
Androderm therapy has not been evaluated in women and must not be used in women. Testosterone may cause fetal harm.
Androderm is contraindicated in patients with known hypersensitivity to any of its components.
WARNINGS
Prolonged use of high doses of orally active 17-alpha-alkyl androgens (e.g., methyltestosterone) has been associated with the development of peliosis hepatis, cholestatic jaundice and hepatic neoplasms, including hepatocellular carcinoma (see PRECAUTIONS, Carcinogenesis). Peliosis hepatis can be a life-threatening or fatal complication. Testosterone is not known to produce these adverse effects.
Geriatric patients treated with androgens may be at an increased risk for the development of prostatic hyperplasia.
Geriatric patients and other patients with clinical or demographic characteristics that are recognized to be associated with an increased risk of prostate cancer should be evaluated for the presence of subclinical or clinical prostate cancer prior to initiation of testosterone replacement therapy, because testosterone therapy may promote the growth of existing subclinical foci of prostate cancer.2
In men receiving testosterone replacement therapy, surveillance for prostate cancer should be consistent with current practices for eugonadal men (see PRECAUTIONS, Carcinogenesis).
Edema, with or without congestive heart failure, may be a serious complication of androgen treatment in patients with preexisting cardiac, renal, or hepatic disease. In addition to discontinuation of the drug, diuretic therapy may be required.
Gynecomastia frequently develops and occasionally persists in patients being treated for hypogonadism.
PRECAUTIONS
General
The physician should instruct patients to report any of the following side effects of androgens:
-
Too frequent or persistent erections of the penis
-
Any nausea, vomiting, jaundice, or ankle swelling
Virilization of female sexual partners has been reported with male use of a topical testosterone solution. Topically applied creams leave as much as 90 mg residual testosterone on the skin. The occlusive backing film on Androderm (testosterone transdermal system) prevents the partner from coming in contact with the active material in the system. Transfer of the system to the partner is unlikely.
Changes in body hair distribution, significant increase in acne, or other signs of virilization of the female partner should be brought to the attention of a physician.
Information for patients
An information brochure is available for patients concerning the use of Androderm.
Advise patients of the following:
Androderm should not be applied to the scrotum.
Androderm should not be applied over a bony prominence or on a part of the body that could be subject to prolonged pressure during sleep or sitting. Application to these sites has been associated with burn-like blister reactions.
Androderm does not have to be removed during sexual intercourse, nor while taking a shower or bath.
Androderm systems should be applied nightly.
Skin burns have been reported at the patch site in several patients wearing an aluminized transdermal system during a magnetic resonance imaging scan (MRI). Because Androderm contains aluminum, it is recommended to remove the system before undergoing an MRI.
Laboratory tests
Hemoglobin and hematocrit should be checked periodically to detect polycythemia in patients who are receiving androgen therapy.
Liver function, prostate specific antigen, total cholesterol and HDL cholesterol should be checked periodically.
Drug interactions
Anticoagulants: C-17 substituted derivatives of testosterone, such as methandrostenolone, have been reported to decrease the anticoagulant requirements of patients receiving oral anticoagulants. Patients receiving oral anticoagulants require close monitoring especially when androgens are started or stopped.
Oxyphenbutazone: Concurrent administration of oxyphenbutazone and androgens may result in elevated serum levels of oxyphenbutazone.
Insulin: In diabetic patients, the metabolic effects of androgens may decrease blood glucose and, therefore, insulin requirements.
Drug/laboratory test interactions
Androgens may decrease levels of thyroxine-binding globulin, resulting in decreased total T4 serum levels and increased resin uptake of T3 and T4. Free thyroid hormone levels remain unchanged, however, and there is no clinical evidence of thyroid dysfunction.
Carcinogenesis, mutagenesis, impairment of fertility
Animal Data: Testosterone has been tested by subcutaneous injection and implantation in mice and rats. The implant induced cervical-uterine tumors in mice, which metastasized in some cases. There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver in rats.
Human Data: There are rare reports of hepatocellular carcinoma in patients receiving long-term therapy with androgens in high doses. Withdrawal of drugs did not lead to regression of the tumors in all cases.
Geriatric patients treated with androgens may be at an increased risk for the development of prostatic hyperplasia.
Geriatric patients and other patients with clinical or demographic characteristics that are recognized to be associated with an increased risk of prostate cancer should be evaluated for the presence of subclinical or clinical prostate cancer prior to initiation of testosterone replacement therapy, because testosterone therapy may promote the growth of existing subclinical foci of prostate cancer.2
In men receiving testosterone replacement therapy, surveillance for prostate cancer should be consistent with current practices for eugonadal men.
Pregnancy
Category X: (See Contraindications).
Teratogenic effects
Androderm must not be used in women.
Nursing mothers
Androderm must not be used in women.
Pediatric use
Androderm has not been evaluated clinically in males under 15 years of age.
ADVERSE REACTIONS
Adverse Events Associated with Androderm (testosterone transdermal system)
In clinical studies of 122 patients treated with Androderm, the most common adverse events reported were skin reactions at the site of system application. Transient mild to moderate erythema was observed at the site of application in the majority of patients at some time during treatment.
The adverse reactions reported by more than 1% of patients are listed below shown in order of decreasing frequency.
| Event | Percent of Patients |
| pruritus at application site | 37% |
| burn-like blister reaction under system | 12% |
| erythema at application site | 7% |
| vesicles at application site | 6% |
| prostate abnormalities | 5% |
| headache | 4% |
| allergic contact dermatitis to the system | 4% |
| burning at application site | 3% |
| induration at application site | 3% |
| depression | 3% |
| rash | 2% |
| gastrointestinal bleeding | 2% |
The following reactions occurred in less than 1% of patients: fatigue; body pain; pelvic pain; hypertension; peripheral vascular disease; increased appetite; accelerated growth; anxiety; confusion; decreased libido; paresthesia; thinking abnormalities; vertigo; acne; bullae at application site; mechanical irritation at application site; rash at application site; contamination of application site; prostate carcinoma; dysuria; hematuria; impotence; urinary incontinence; urinary tract infection; testicular abnormalities.
Three types of application site reactions occurred: irritation which included mild to moderate erythema, induration or burning; allergic contact dermatitis; and burn-like blister reactions.
Chronic skin irritation caused 5% of patients to discontinue treatment. Mild skin irritation may be ameliorated by treatment of affected skin with over-the-counter topical hydrocortisone cream applied after system removal.
Applying a small amount of 0.1% triamcinolone acetonide cream (Rx) to the skin under the central drug reservoir of the Androderm system has been shown to reduce the incidence and severity of skin irritation. The administration of 0.1% triamcinolone acetonide cream (Rx) does not significantly alter transdermal absorption of testosterone from the system. Ointment formulations should not be used for pretreatment as they may significantly reduce testosterone absorption.
Five patients (4%) developed allergic contact dermatitis after 3 to 8 weeks treatment that required discontinuation. These reactions were characterized by pruritus, erythema, induration and in some instances vesicles or bullae, which recurred with each system application. Rechallenge with components of the system showed ethanol sensitization in 4 patients. One patient’s reaction was attributed to testosterone. None of these patients had adverse sequelae related to oral alcohol ingestion or to injectable testosterone use. Older patients may be more prone to develop allergic contact dermatitis.
Fourteen patients (12%) had burn-like blister reactions that involved bullae, epidermal necrosis or the development of ulcerated lesions. These reactions typically occurred once, at a single application site; 5 patients experienced a single recurrence. None withdrew from the clinical trials. These reactions occurred at a rate of approximately 1 in 6,500 system applications (1 in 3,250 treatment days). The majority of these lesions were associated with system application over bony prominences or on parts of the body that may have been subject to prolonged pressure during sleep or sitting (e.g., over the deltoid region of the upper arm, the greater trochanter of the femur, or the ischial tuberosity). The more severe lesions healed over several weeks with scarring in some cases. Such lesions should be treated as burns.
Adverse Events Associated with Injection or Oral Treatments
Skin and Appendages: Hirsutism, male pattern of baldness, seborrhea, and acne.
Endocrine and Urogenital: Gynecomastia and excessive frequency and duration of penile erections. Oligospermia may occur at high dosages (see CLINICAL PHARMACOLOGY).
Fluid and Electrolyte Disturbances: Retention of sodium, chloride, water, potassium, calcium, and inorganic phosphates.
Gastrointestinal: Nausea, cholestatic jaundice, alterations in liver function tests. Rare instances of hepatocellular neoplasms and peliosis hepatis have occurred (see WARNINGS).
Hematologic: Suppression of clotting factors II, V, VII, and X; bleeding in patients on concomitant anticoagulant therapy and polycythemia.
Nervous System: Increased or decreased libido, headache, anxiety, depression and generalized paresthesia.
Metabolic: Increased serum cholesterol.
Miscellaneous: Rarely, anaphylactoid reactions.
DRUG ABUSE AND DEPENDENCE
Androderm (testosterone transdermal system) is a Schedule III controlled substance under the Anabolic Steroids Control Act.
Oral consumption of the Androderm system or the gel contents of the system will not result in clinically significant serum testosterone concentrations in the target organs due to extensive first-pass metabolism.
OVERDOSAGE
There is one report of acute overdosage with testosterone enanthate injection: testosterone levels of up to 11,400 ng/dL were implicated in a cerebrovascular accident.
DOSAGE AND ADMINISTRATION
The usual starting dose is one Androderm 5 mg system or two Androderm 2.5 mg systems applied nightly for 24 hours, providing a total dose of 5 mg/day.
The adhesive side of the Androderm system should be applied to a clean, dry area of the skin on the back, abdomen, upper arms, or thighs. Avoid application over bony prominences or on a part of the body that may be subject to prolonged pressure during sleep or sitting (e.g., the deltoid region of the upper arm, the greater trochanter of the femur, and the ischial tuberosity). DO NOT APPLY TO THE SCROTUM. The sites of application should be rotated, with an interval of 7 days between applications to the same site. The area selected should not be oily, damaged, or irritated. (See Table 2.)
The system should be applied immediately after opening the pouch and removing the protective release liner. The system should be pressed firmly in place, making sure there is good contact with the skin, especially around the edges.
To ensure proper dosing, the morning serum testosterone concentration should be measured following system application the previous evening. If the serum concentration is outside the normal range, sampling should be repeated with assurance of proper system adhesion as well as appropriate application time. Confirmed serum concentrations outside the normal range may require increasing the daily dose to 7.5 mg (i.e., one 5 mg and one 2.5 mg systems or three 2.5 mg systems) or decreasing the daily dose to 2.5 mg (i.e., one 2.5 mg system), maintaining nightly application. Because of variability in analytical values among diagnostic laboratories, this laboratory work and any later analyses for assessing the effect of Androderm therapy, should be performed at the same laboratory so results can be compared.
Mild skin irritation may be ameliorated by treatment of the affected skin with over-the-counter topical hydrocortisone cream applied after system removal.
Applying a small amount of 0.1% triamcinolone acetonide cream (Rx) to the skin under the central drug reservoir of the Androderm system has been shown to reduce the incidence and severity of skin irritation. The administration of 0.1% triamcinolone acetonide cream (Rx) does not significantly alter transdermal absorption of testosterone from the system. Ointment formulations should not be used for pretreatment as they may significantly reduce testosterone absorption.
Androderm (testosterone transdermal system) therapy for non-virilized patients may be initiated with one 2.5 mg/day system applied nightly.
HOW SUPPLIED
Androderm (testosterone transdermal system) 2.5 mg/day.
Each system contains 12.2 mg testosterone USP for delivery of 2.5 mg of testosterone per day (see DESCRIPTION).
Cartons of 60 systems NDC 52544-469-60
Androderm (testosterone transdermal system) 5 mg/day.
Each system contains 24.3 mg testosterone USP for delivery of 5 mg of testosterone per day (see DESCRIPTION).
Cartons of 30 systems NDC 52544-470-30
Storage and Disposal
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F). Apply to skin immediately upon removal from the protective pouch. Do not store outside the pouch provided. Damaged systems should not be used. The drug reservoir may be burst by excessive pressure or heat. Discard systems in household trash in a manner that prevents accidental application or ingestion by children, pets or others.
REFERENCES
-
Mazer NA, et al. Mimicking the circadian pattern of testosterone and metabolite levels with an enhanced transdermal delivery system. In Gurney, Junjinger, Peppas, eds. Pulsatile Drug Delivery: Current Applications and Future Trends. Stuttgart: Wiss. Verl.-Ges.; 1993, 73-97.
-
Schroeder FH. Androgens and carcinoma of the prostate. In Neischlag E, Behre HM, eds. Testosterone Action, Deficiency, Substitution. Berlin/Heidelberg: Springer-Verlag; 1990, 245-260.
Rx only
Revised: November 2005
© Watson Pharma, Inc., 1999
U.S. Patent Nos. 4,849,224, 4,855,294, 4,863,970, 4,983,395, 5,152,997, and 5,164,190.
Watson Pharma, Inc.
A subsidiary of Watson Pharmaceuticals, Inc.
Corona, CA 92880 USA
| Androderm (Testosterone) | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
| Androderm (Testosterone) | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
Revised: 09/2006