BENZEFOAM
-
benzoyl peroxide aerosol, foam
Onset Therapeutics, LLC
----------
FOR EXTERNAL USE ONLY
Rx Only
DESCRIPTION
Each gram of BENZEFOAM™ Emollient Foam contains 5.3% benzoyl peroxide in an aqueous based emollient foam vehicle containing BHT, C12-15 alkyl benzoate, cetearyl alcohol, citric acid, dimethicone, disodium EDTA, emulsifying wax, glycerin, methylparaben, propylene glycol, propylparaben, purified water, sodium citrate, steareth-10. Also contains: Propellant HFA-134A (1,1,1,2-tetrafluoroethane).
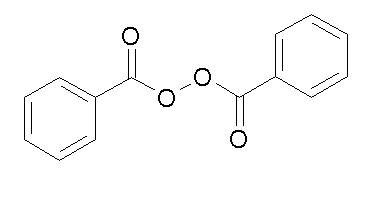
Benzoyl peroxide is an oxidizing agent that possesses antibacterial properties and is classified as a keratolytic. Benzoyl peroxide (C14H10O4) is represented by the following structure:
CLINICAL PHARMACOLOGY
The exact method of action of benzoyl peroxide in acne vulgaris is not known. Benzoyl peroxide is an antibacterial agent with demonstrated activity against Propionibacterium acnes. This action, combined with the mild keratolytic effect of benzoyl peroxide, is believed to be responsible for its usefulness in acne. Benzoyl peroxide is absorbed by the skin where it is metabolized to benzoic acid and excreted as benzoate in the urine.
INDICATIONS AND USAGE
BENZEFOAM Emollient Foam is indicated for use in the topical treatment of mild to moderate acne vulgaris.
CONTRAINDICATIONS
BENZEFOAM Emollient Foam should not be used in patients who have shown hypersensitivity to benzoyl peroxide or to any of the other ingredients in the product. Discontinue use if hypersensitivity is observed.
WARNINGS
FOR EXTERNAL USE ONLY. Not For Ophthalmic Use. When using this product, avoid unnecessary sun exposure and use a sunscreen. Keep out of the reach of children. Contents under pressure. Do not puncture or incinerate container. Do not expose to temperatures above 120°F (49°C).
PRECAUTIONS
General
If severe irritation develops, discontinue use and institute appropriate therapy.
Information for Patients
This medication is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. Avoid contact with eyes, eyelids, lips, and mucous membranes. If accidental contact occurs, rinse with water. If excessive redness or irritation develops, discontinue use and consult your physician. AVOID CONTACT WITH HAIR, FABRICS OR CARPETING AS BENZOYL PEROXIDE WILL CAUSE BLEACHING OR DICOLORATION.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Based upon all available evidence, benzoyl peroxide is not considered to be a carcinogen. However, data from a study using mice known to be highly susceptible to cancer suggest that benzoyl peroxide acts as a tumor promoter. The clinical significance of the findings is not known.
Pregnancy
Category C. Animal reproduction studies have not been conducted with benzoyl peroxide. It is also not known whether benzoyl peroxide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzoyl peroxide should be used by a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in the human milk. Because many drugs are excreted in human milk, caution should be exercised when benzoyl peroxide is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in children below the age of 12 have not been established.
ADVERSE REACTIONS
Allergic contact dermatitis and dryness have been reported with topical benzoyl peroxide therapy.
OVERDOSAGE
If excessive scaling, erythema or edema occurs, the use of this preparation should be discontinued. To hasten resolution of the adverse effects, cool compresses may be used. After symptoms and signs subside, a reduced dosage schedule may be cautiously tried if the reaction is judged to be due to excessive use and not allergenicity.
DOSAGE AND ADMINISTRATION
For Proper Dispensing of Foam, Shake Vigorously and Tap Bottom of Can Before Each Use. Prime Container Before Initial Use: Shake Vigorously. After shaking, gently tap bottom of can onto palm of other hand or a solid surface at least 3 times. Remove cap. Hold can upright over sink. Avoid contact with hair, fabrics or carpeting as benzoyl peroxide will cause bleaching or discoloration. Firmly depress the actuator for 1 to 3 seconds until tab breaks and foam begins to dispense. (If foam does not dispense within 3 seconds: repeat entire process and depress the actuator again until foam begins to dispense.)
Before Each Use: Shake vigorously and gently tap bottom of can onto palm of other hand or a solid surface at least 3 times.
During Use: Holding can upright, dispense BENZEFOAM Emollient Foam into palm of hand and apply to affected area once daily, or as directed by a physician. For facial acne, use a dollop the size of a marble. For acne on either the back or chest, use a dollop the size of a whole walnut. Rub in until completely absorbed. Wipe off any excess foam from actuator after use. Wash hands with soap and water after applying BENZEFOAM Emollient Foam.
Frequency of use should be adjusted to obtain the desired clinical response. Gentle cleansing of the affected areas prior to application of BENZEFOAM Emollient Foam may be beneficial.
HOW SUPPLIED
BENZEFOAM Emollient Foam is supplied in a 60g (NDC 16781-194-60) aluminum can.
Store at room temperature: 59° - 77°F (15° - 25°C).
Protect from freezing.
Store upright.
Manufactured in USA For:

Onset Therapeutics
Cumberland, RI 02864
www.onsettx.com
888-713-8154
PATENT PENDING
P/N 2616-pf Rev. 0

PATIENT INFORMATION
|
IMPORTANT: For use on skin only (topical use). When using this product, avoid unnecessary sun exposure and use a sunscreen. Avoid contact with hair, fabrics or carpeting as benzoyl peroxide will cause bleaching or discoloration. Contents under pressure. Do not puncture or incinerate container. |
Read the Patient Information that comes with BENZEFOAMTM Emollient Foam before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of speaking with your doctor about your medical condition or your treatment.
WHAT IS BENZEFOAM EMOLLIENT FOAM?
BENZEFOAM Emollient Foam is a prescription medicine used on the skin (topical) to treat mild to moderate acne vulgaris. BENZEFOAM Emollient Foam contains benzoyl peroxide.
WHO SHOULD NOT USE BENZEFOAM EMOLLIENT FOAM?
Do not use BENZEFOAM Emollient Foam if you have shown hypersensitivity to benzoyl peroxide or to any of the other ingredients in the product.
WHAT SHOULD I TELL MY DOCTOR BEFORE USING BENZEFOAM EMOLLIENT FOAM?
Before using BENZEFOAM Emollient Foam, tell your doctor about all of your medical conditions, including if you:
- have any allergies.
- are pregnant or planning to become pregnant. It is not known if BENZEFOAM Emollient Foam will harm your unborn baby.
- are breastfeeding or plan to breast-feed. It is not known if BENZEFOAM Emollient Foam passes into your breast milk.
Tell your doctor about all the medicines and skin products you use. Other skin and topical acne products may increase the irritation of your skin when used with BENZEFOAM Emollient Foam. Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist when you get a new medicine.
HOW SHOULD I USE BENZEFOAM EMOLLIENT FOAM?
- Use BENZEFOAM Emollient Foam exactly as prescribed.
- Your doctor will tell you how long to use BENZEFOAM Emollient Foam.
- Throw away (discard) any unused BENZEFOAM Emollient Foam after its expiration date.
INSTRUCTIONS FOR APPLYING BENZEFOAM EMOLLIENT FOAM
- Before you apply BENZEFOAM Emollient Foam, wash your face gently with a mild soap, rinse with warm water, and pat your skin dry.
- For Proper Dispensing of Foam, Shake Vigorously and Tap Bottom of Can Before Each Use.
- Prime Container Before Initial Use: Shake vigorously. After shaking, gently tap bottom of can onto palm of other hand or a solid surface at least 3 times. Remove cap. Hold can upright over sink. Avoid contact with hair, fabrics or carpeting as benzoyl peroxide will cause bleaching or discoloration. Firmly depress the actuator for 1 to 3 seconds until tab breaks and foam begins to dispense. (If foam does not dispense within 3 seconds: repeat entire process and depress the actuator again until foam begins to dispense.)
- Before Each Use: Shake vigorously and gently tap bottom of can onto palm of other hand or a solid surface at least 3 times.
- During Use: Holding can upright, dispense BENZEFOAM Emollient Foam into palm of hand and apply to affected area once daily, or as directed by a physician. For facial acne, use a dollop the size of a marble. For acne on either the back or chest, use a dollop the size of a whole walnut. Rub in until completely absorbed. Wipe off any excess foam from actuator after use.
- Caution:BENZEFOAM Emollient Foam may bleach hair or colored fabric.
- Wash your hands with soap and water after applying BENZEFOAM Emollient Foam.
- If BENZEFOAM is applied to the chest or back, wearing a white t-shirt may avoid bleaching of clothing.
- Avoid getting BENZEFOAM Emollient Foam in your mouth, eyes, or nose, or on your lips. If this occurs, rinse the affected area with warm water.
- Do not get BENZEFOAM Emollient Foam on cuts or open wounds.
- Do not use BENZEFOAM Emollient Foam more frequently than prescribed by your doctor.
WHAT SHOULD I AVOID WHILE USING BENZEFOAM EMOLLIENT FOAM?
- Limit your time in sunlight. Avoid using tanning beds or sun lamps. If you have to be in sunlight, wear a wide-brimmed hat or other protective clothing, and a sunscreen with SPF 15 rating or higher. Your doctor can give you more information about why this is important.
- Do not wash your face more than 2 to 3 times a day. Washing your face too often or scrubbing it may make your acne worse.
WHAT ARE POSSIBLE SIDE EFFECTS WITH BENZEFOAM EMOLLIENT FOAM?
Side effects with BENZEFOAM Emollient Foam include:
- Skin irritation. Stop using BENZEFOAM Emollient Foam and call your doctor if you have a skin rash or your skin becomes very red, itchy or swollen. Talk to your doctor about any side effect that bothers you or that does not go away.
This is not the only possible side effect with BENZEFOAM Emollient Foam. Call your doctor for medical advice about side effects. You may report side effects to Onset Therapeutics at 1-888-713-8154.
HOW SHOULD I STORE BENZEFOAM EMOLLIENT FOAM?
Store at room temperature: 59º - 77ºF (15º - 25ºC).
Do not expose to temperatures above 120°F (49°C)
Protect from freezing.
Store upright.
Keep BENZEFOAM Emollient Foam and all medicines out of the reach of children.
GENERAL INFORMATION ABOUT BENZEFOAM EMOLLIENT FOAM
Medicines are sometimes prescribed for conditions that are not mentioned in Patient Information leaflets. Do not use BENZEFOAM for a condition for which it was not prescribed. Do not give BENZEFOAM Emollient Foam to other people, even if they have the same condition you have. This leaflet summarizes the most important information about BENZEFOAM Emollient Foam. If you would like more information, talk with your doctor. You can also ask your doctor or pharmacist for information about BENZEFOAM Emollient Foam that is written for healthcare professionals. For more information about BENZEFOAM Emollient Foam, call 1-888-713-8154 or go to www.BenzeFoam.com.
WHAT ARE THE INGREDIENTS IN BENZEFOAM EMOLLIENT FOAM?
Active Ingredients: benzoyl peroxide 5.3%
Inactive Ingredients: BHT, C12-15 alkyl benzoate, cetearyl alcohol, citric acid, dimethicone, disodium EDTA, emulsifying wax, glycerin, methylparaben, propylene glycol, propylparaben, purified water, sodium citrate, steareth-10. Also contains: Propellant HFA-134a (1,1,1,2-tetrafluoroethane).
Manufactured in USA For:

Onset Therapeutics
Cumberland, RI 02864
www.onsettx.com
888-713-8154
PATENT PENDING
P/N 2616-pf Rev. 0

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Box of six 5 Gram Cans
NDC 16781-194-06
Rx Only
BenzEFoamTM
EmollientFoam
benzoyl peroxide 5.3%
Delivered in DelevoTM Foam
The Science of ComplianceTM
For topical treatment of mild to moderate acne vulgaris
See enclosed full Prescribing Information
Onset THERAPEUTICS
Professional Samples
Enclosed: Six 5g Samples
Available in 60g Cans

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Can Label, 5 Grams
NDC16781-194-06
Rx Only
Professional Sample
Not for Sale
Net Weight 5g
BenzEFoamTM
EmollientFoam
benzoyl peroxide 5.3%
Delivered in DelevoTM Foam
The Science of ComplianceTM
Manufactured in USA For:
Onset Therapeutics
Cumberland, RI 02864
(888) 713-8154
www.onsettx.com
Expiration Date and Lot Code on bottom of can
PATENT PENDING P/N 2135 Rev. 0
Sample may not dispense entire contents.

| BENZEFOAM
benzoyl peroxide aerosol, foam |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 09/18/2009 | ||
| Labeler - Onset Therapeutics, LLC (793223707) |
| Registrant - Onset Therapeutics, LLC (964275155) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Onset Therapeutics, LLC | 793223707 | Manufacture | |