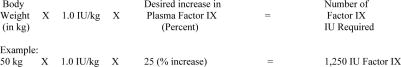PROFILNINE SD
-
factor ix complex
Grifols Biologicals Inc.
----------
Factor IX Complex
Profilnine® SD
Solvent Detergent Treated
DESCRIPTION
Factor IX Complex, Profilnine® SD, Solvent
Detergent Treated, is a sterile, lyophilized concentrate of Factor IX
(antihemophilic factor B), Factor II (prothrombin), Factor X
(Stuart-Prower Factor), and low levels of Factor VII (proconvertin)
derived from human plasma. Factor II content has been assayed at no more
than (NMT) 150 Units per 100 Factor IX Units, Factor X at NMT 100 Units
per 100 Factor IX Units, and Factor VII at NMT 35 Units per 100 Factor
IX Units. Profilnine SD is intended for intravenous
administration only. Each vial is a single dose container.
Profilnine SD is a non-activated Factor IX
Complex prepared from pooled human plasma and purified by DEAE cellulose
adsorption. Profilnine SD is treated with a mixture
of the organic solvent tri(n-butyl)phosphate (TNBP) and the nonionic
detergent polysorbate 80 (Solvent Detergent Mixture) to reduce risks of
transmission of viral infection. However, no procedure has been shown to
be totally effective in removing viral infectivity from coagulation
factor products. Each vial of Profilnine SD is
labeled with the Factor IX potency expressed in International Units
(IU). Profilnine SD does not contain heparin.
Profilnine SD contains low levels of activated
coagulation factors, as indicated by the non-activated Partial
Thromboplastin Time Test.1,2 Profilnine
SD contains no preservatives.
When reconstituted with the appropriate volume of Sterile Water
for Injection, USP, Profilnine SD contains not more
than 2.5 μg polysorbate 80 and 0.40 μg TNBP per IU of
Factor IX.
CLINICAL PHARMACOLOGY
Profilnine SD is a mixture of vitamin
K-dependent clotting factors. The administration of Factor IX Complex,
Profilnine SD, temporarily increases the plasma
levels of Factor IX, thus minimizing the hazards of hemorrhage. A
clinical study, which evaluated twelve subjects with hemophilia B,
indicated that, following administration of
Profilnine SD, Factor IX in vivo half-life is 24.68 ±
8.29 hours and recovery is 1.15 ± 0.16 IU/dL per IU infused per
kg body weight.3
Administration of Factor IX Complex can result in higher than
normal levels of Factor II due to its significantly longer
half-life.4
The retrovirus known as Human Immunodeficiency Virus (HIV-1) has
been identified as the causative agent of Acquired Immunodeficiency
Syndrome (AIDS) and has been shown to be transmissible via blood or
blood products. The solvent detergent process used in the manufacture of
Profilnine SD has been shown to provide a very
high level of virus kill without compromising protein structure and
function.5 The susceptibility of human pathogenic viruses
such as HIV-1, hepatitis B virus, hepatitis C virus and marker viruses
such as Sindbis and Vesicular Stomatitis Virus (VSV) to inactivation by
organic solvent detergent treatment has been discussed in the
literature.6-8
The solvent detergent process used in the manufacture of
Profilnine SD was shown to inactivate greater
than 12.2 logs of HIV-1 when the retrovirus was intentionally added to
product samples under laboratory evaluation (as measured by virus
antigen capture and reverse transcriptase assays). In addition, this
process was shown to inactivate 6.0 logs of HIV-2 (as measured by
reverse transcriptase assays) when the retrovirus was intentionally
added to product samples. In order to assess the ability of the solvent
detergent process to inactivate other viruses such as hepatitis B and C
virus, the inactivation of the model viruses, Sindbis virus and
vesicular stomatitis virus (VSV), by solvent detergent treatment was
studied. Prior to solvent detergent treatment, samples were inoculated
with a titer of either Sindbis or VSV. The results demonstrated that a
minimum of 5.3 logs of Sindbis and a minimum of 4.9 logs of VSV were
removed after 180 minutes of incubation with solvent detergent (when
compared to an untreated control). It should be noted that the
incubation time in the actual Profilnine SD process
is twice (360 minutes total) that used in the model virus studies.
The ability of the Profilnine SD process to
eliminate virus, by physically partitioning virus from product, was
evaluated at the DEAE chromatography step. Addition of Sindbis virus
prior to Factor IX Complex adsorption by DEAE chromatography showed this
step to eliminate 1.4 logs of added virus.
However, no treatment method has yet been shown capable of
totally eliminating all potential infective virus in preparations of
coagulation factor concentrates.
INDICATIONS AND USAGE
Factor IX Complex, Profilnine SD is
indicated for the prevention and control of bleeding in patients with
Factor IX deficiency due to hemophilia B.
This product contains non-therapeutic levels of Factor VII, and
is not indicated for use in the
treatment of Factor VII deficiency.
CONTRAINDICATIONS
None known.
WARNINGS
Because Factor IX Complex, Profilnine SD is
made from pooled human plasma, it may carry a risk of transmitting
infectious agents, e.g., viruses, and theoretically, the
Creutzfeldt-Jakob disease (CJD) agent. Stringent procedures designed to
reduce the risk of adventitious agent transmission have been employed in
the manufacture of this product, from the screening of plasma donors and
the collection and testing of plasma to the application of viral
elimination/reduction steps such as DEAE chromatography and solvent
detergent treatment in the manufacturing process.9-10 Despite
these measures, such product can potentially transmit disease, therefore
the risk of infectious agents cannot be totally eliminated. The
physician should weigh the risks and benefits of the use of this product
and should discuss these with the patient.
Individuals who receive infusions of blood or plasma products may
develop signs and/or symptoms of some viral infections. Scientific
opinion encourages hepatitis B and hepatitis A vaccinations for patients
with hemophilia at birth or diagnosis.
In patients undergoing surgery and in patients with known liver
disease, thrombosis or disseminated intravascular coagulation (DIC) are
serious and potentially fatal adverse reactions associated with the
administration of Factor IX Complex concentrates.11-13
Infrequent but consistent reports have been described which
indicate that patients are at greater risk of developing thrombosis and
DIC in the period following surgery. Cases have also been cited which
indicate that patients with liver disease may be predisposed to
thrombosis or DIC when treated with Factor IX Complex. Although the
available data is limited, Profilnine SD should
only be administered to patients when the beneficial effects of use
outweigh the serious risk of potential hypercoagulation.
PRECAUTIONS
General
Factor IX Complex, Profilnine SD
should not be
administered at a rate exceeding 10 mL/minute. Rapid
administration may result in vasomotor reactions.
Nursing personnel, and others who administer this
material, should exercise appropriate caution in handling due to
the risk of exposure to viral infection.
Discard any unused contents. Discard administration
equipment after single use. Do not resterilize components. Do not reuse components.
Information for Patients
Patients should be informed of the early symptoms and
signs of hypersensitivity reaction, including hives, generalized
urticaria, chest tightness, dyspnea, wheezing, faintness,
hypotension, and anaphylaxis. Patients should be advised to
discontinue use of the product and contact their physician
and/or seek immediate emergency care, depending on the severity
of the reaction, if these symptoms occur.
Some viruses, such as parvovirus B19 or hepatitis A, are
particularly difficult to remove or inactivate at this time.
Parvovirus B19 may most seriously affect sero-negative pregnant
women, or immunocompromised individuals. The majority of
parvovirus B19 and hepatitis A infections are acquired by
environmental (natural) sources.
Pregnancy Category C
Animal reproduction studies have not been conducted with
Profilnine SD. It is also not known
whether Profilnine SD can cause fetal harm
when administered to a pregnant woman or can affect reproduction
capacity. Profilnine SD should be given to
a pregnant woman only if clearly indicated.
Pediatric Use
Clinical Trials for safety and effectiveness in pediatric
patients 16 years of age and younger have not been conducted.
Across a well controlled half-life and recovery clinical trial
in patients previously treated with factor IX concentrates for
Hemophilia B, the two pediatric patients receiving
Profilnine SD (solvent detergent
treated) responded similarly when compared with the adult
patients. There were no adverse events in the pediatric patients
and one mild adverse event in the adult population (headache).
Anecdotal evaluation of the results indicate no safety and
efficacy differences between pediatric and adult
populations.3
ADVERSE REACTIONS
Adverse reactions characterized by either thrombosis or
disseminated intravascular coagulation (DIC) are associated with
administration of Factor IX Complex concentrates.11-14 In
particular, patients who receive prolonged treatment with Factor IX
Complex concentrates postoperatively or with known liver disease should
be kept under close observation for signs or symptoms of intravascular
coagulation. Continued administration should be left to the discretion
of the physician.
Adverse reactions may include urticaria, fever, chills, nausea,
vomiting, headache, somnolence, lethargy, flushing or tingling. For most
reactive individuals, slowing the rate of infusion relieves the
symptoms. For those highly reactive individuals, a different lot may be
satisfactory.
To report SUSPECTED ADVERSE REACTIONS, contact Grifols at
1-888-GRIFOLS (1-888-474-3657) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DOSAGE AND ADMINISTRATION
For adult usage:
Factor IX Complex, Profilnine SD
should be administered intravenously, promptly following
reconstitution with the supplied diluent. Although
Profilnine SD is stable for at least
three (3) hours at room temperature after reconstitution, prompt
administration is recommended to avoid the ill effect of any
inadvertent bacterial contamination occurring during
reconstitution. Profilnine SD may be
administered by injection (plastic disposable syringe only) or
infusion. Administer at room temperature, do not refrigerate
after reconstitution and discard any unused contents.
Each vial of Profilnine SD is
labeled with the total units expressed as International Units
(IU) which is referenced to the WHO International Standard. One
unit approximates the activity in one mL of normal plasma.
A 1.0% increase in Factor IX (0.01 IU)/IU administered/kg
can be expected.15 The amount of
Profilnine SD required to establish
hemostasis will vary with each patient and depend on the
circumstances. The following formula may be used as a guide in
determining the number of units to be administered:
In normal clinical practice there is variability among
patients and their clinical condition.
Therefore, the Factor IX level of each patient should be
monitored frequently during replacement therapy.
Mild to moderate hemorrhages may usually be treated with
a single administration sufficient to raise the plasma Factor IX
level to 20 to 30 percent. In the event of more serious
hemorrhage, the patient's plasma Factor IX level should
be raised to 30 to 50 percent. Infusions are generally required
daily.
Surgery in patients with Factor IX deficiency requires
that the Factor IX level should be raised to 30 to 50 percent
for at least one week following operation. For dental
extractions, the Factor IX level should be raised to 50 percent
immediately prior to the procedure; additional Factor IX Complex
may be given if bleeding recurs.
For pediatric usage: See PRECAUTIONS
RECONSTITUTION
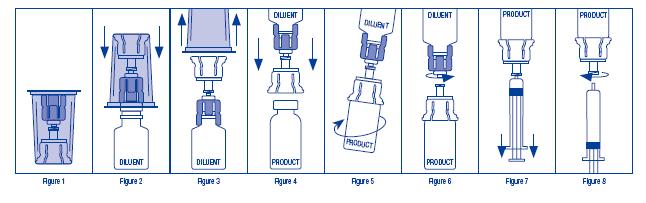
Use Aseptic Technique
- Warm diluent (Sterile Water for Injection, USP) and
concentrate (Profilnine SD) to at least
room temperature (but not above 37 °C).
- Remove the plastic flip off cap from the diluent vial.
- Gently swab the exposed stopper surface with a cleansing agent
such as alcohol trying to avoid leaving any excess cleansing agent on the stopper.
- Open the Mix2Vial® package by peeling away the lid (Figure 1). Leave the Mix2Vial in
the clear outer packaging.
- Place the diluent vial upright on an even surface and hold the vial tight and pick up
the Mix2Vial in its clear outer packaging. Holding the diluent vial securely, push the
blue end of the
Mix2Vial vertically down through the diluent vial stopper (Figure 2).
- While holding onto the diluent vial, carefully remove the clear outer packaging from the
Mix2Vial set, ensuring the Mix2Vial remains attached to the diluent vial (Figure 3).
- Place the product vial upright on an even surface, invert the diluent vial with the Mix2Vial attached.
- While holding the product vial securely on a flat surface, push the
clear end of the Mix2Vial set
vertically down through the product vial stopper (Figure 4). The diluent will automatically transfer
out of its vial into the product vial. (NOTE: If the Mix2Vial is connected at an angle, the vacuum
may be released from the product vial and the diluent will not transfer into the product vial.)
- With the diluent and product vials still attached to the Mix2Vial, gently swirl the product vial to ensure the
product is fully dissolved (Figure 5). Reconstitution requires less than 10 minutes. Do not shake the vial.
- Disconnect the Mix2Vial into two separate pieces (Figure 6) by holding each vial adapter and twisting
counterclockwise. After separating, discard the diluent vial with the
blue end of the Mix2Vial.
- Draw air into an empty, sterile syringe. Keeping the product vial upright with the clear
end of the Mix2Vial attached, screw
the disposable syringe onto the luer lock portion of the Mix2Vial device by pressing and twisting clockwise. Inject air into the product vial.
- While keeping the syringe plunger depressed, invert the system upside down and draw the reconstituted product into the syringe by pulling
the plunger back slowly (Figure 7).
- When the reconstituted product has been transferred into the syringe, firmly hold the barrel of the syringe and the clear vial
adapter (keeping the syringe plunger facing down) and unscrew the syringe from the Mix2Vial (Figure 8). Hold the syringe upright
and push the plunger until no air is left in the syringe. Attach the syringe to a venipuncture set.
- NOTE: If the same patient is to receive more than one vial of concentrate, the contents of two vials may be drawn into the
same syringe through a separate unused Mix2Vial set before attaching to the venipuncture set.
- Use the prepared drug as soon as possible within three hours after reconstitution.
- After reconstitution, parenteral drug products should be inspected visually for particulate matter and discoloration prior to
administration, whenever solution and container permit. When reconstitution procedure is strictly followed, a few small particles
may occasionally remain. The Mix2Vial set will remove particles and the labeled potency will not be reduced.
- Discard all administration equipment after use into the appropriate safety container. Do not reuse.
HOW SUPPLIED
Profilnine SD is supplied in sterile
lyophilized form in single dose vials accompanied by a suitable volume
of diluent (Sterile Water for Injection, USP), according to Factor IX
potency. Each vial is labeled with the Factor IX potency expressed in
International Units which is referenced to the WHO International
Standard. Profilnine SD is packaged with a
Mix2Vial filter transfer set for use in administration.
It is available in the following potencies, and the product is
also color coded based upon assay on the carton and vial label as
follows:
| Potency | NDC | Assay Color Code |
| 500 IU FIX/5 mL single dose vial | 68516-3201-1 | 500 IU FIX Range - blue box |
| 1000 IU FIX/10 mL single dose vial | 68516-3202-2 | 1000 IU FIX Range - red box |
| 1500 IU FIX/10 mL single dose vial | 68516-3203-2 | 1500 IU FIX Range - black box |
STORAGE
Profilnine SD should be stored at
temperatures between 2 and 8 °C. Do not freeze diluent.
May be stored at room temperature not to exceed 30 °C
for up to 3 months. When removed from refrigeration, record
the date on the vial or carton.
Rx only
REFERENCES
- Kingdon, H.S., Lundblad, R.L., Veltkamp, J.J., Aronson, D.L.
Potentially thrombogenic materials in Factor IX Concentrates.
Thromb Diath Haemorrh
33:617- 631, 1975.
- Middleton, S.M., Forbes, C.D., Prentice, C.R.M. Thrombogenic
Potential in Factor IX Concentrates Comparison of Tests. Thromb Haemost (Stuttg)
40:574-576, 1979.
- Data on file at Grifols Biologicals Inc.
- Aronson, D.L. Factor IX Complex. Semin Thromb Hemostas 6(1):28-43, 1979.
- Horowitz, B. Investigations into the Application of Tri(n-butyl)
phosphate/Detergent Mixtures to Blood Derivatives. In Viral Inactivation of Plasma
Products, Morgenthaler J.J. (ed), Karger.
- Horowitz, B., Wiebe, M.E. et al. Inactivation of viruses in labile
blood derivatives. Transfusion 25:516-522, 1985.
- Edward, C.A., Piet, M.P.J. et al. Tri(n-butyl)phosphate/detergent
treatment of licensed therapeutic and experimental blood
derivatives. Vox Sang
52:53-59, 1987.
- Prince, A.M., Horowitz, B., Horowitz, M. et al. The development of
virus-free labile blood derivatives-a review. Eur J Epidemiology, 3:103-118,
1987.
- Menache, D., Roberts, H.R. Summary Report and Recommendations of
the Task Force Members and Consultants. Thromb Diath Haemorrh 33:645-647, 1975.
- Carnelli, V., Gomperts, E.D., Friedman, A., et al. Assessment for
Evidence of Non A-Non B Hepatitis in Patients Given
n-Heptane-Suspended Heat-Treated Clotting Factor Concentrate.
Thromb Res, 46:827-834,
1987.
- Lusher, J.M. Management of Hemophiliacs with Inhibitors. Hemophilia in the Child and Adult.
Raven Press, Ltd., New York, 1989, pp. 121-136.
- Aledort, L.M. Factor IX and Thrombosis. Scand J Haematol, Suppl 30:40-42,
1977.
- Kasper, C.K. Thromboembolic Complications. Thromb Diath Haemorrh (Stuttg)
33:640-644, 1975.
- Chistolini, A., Mazzucconi, M.G., Tirindelli, M.L., LaVerde, G.,
Ferrari, A., Mandelli, F. Disseminated intravascular coagulation and
myocardial infarction in a haemophilia B patient during therapy with
prothrombin complex concentrate. Acta
Haematol 83:163-165, 1990.
- Zauber, N.P., Levin, J. Factor IX Levels in Patients with
Hemophilia B (Christmas Disease) Following Transfusion with
Concentrates of Factor IX or Fresh Frozen Plasma (FFP). Medicine 56(3):213-224, 1977.
Manufactured and Distributed by:
Grifols Biologicals Inc.
Los Angeles, CA 90032, U.S.A.
U.S. License No.
1694
DATE OF REVISION: August 2010
Part No.
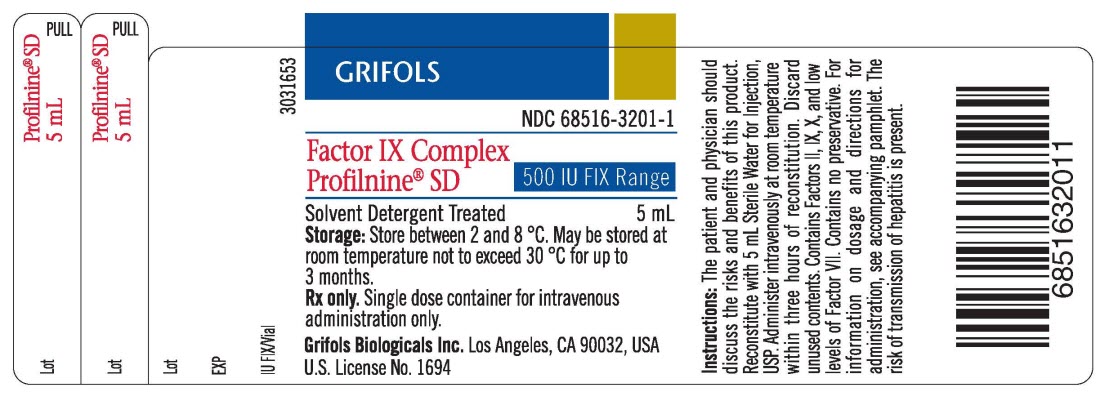
PACKAGE LABEL – PRINCIPAL DISPLAY
PANEL – 500 IU VIAL
GRIFOLS
NDC 68516-3201-1
Factor IX
Complex
Profilnine® SD
MID
Solvent Detergent Treated 5 mL
Storage: Store between 2 and 8
°C. May be stored at room temperature not to exceed 30
°C for up to 3 months.
Rx only. Single dose container for
intravenous administration only.
Grifols Biologicals Inc. Los
Angeles, CA 90032, USA
U.S. License No. 1694
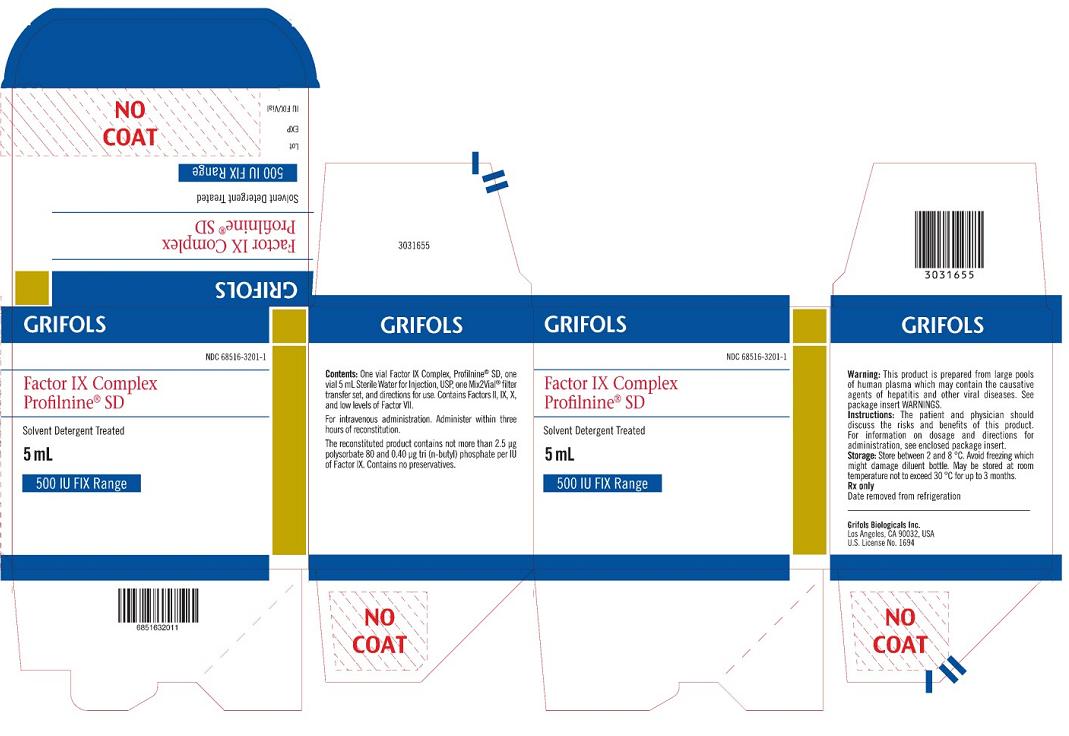
PACKAGE LABEL – PRINCIPAL DISPLAY
PANEL – 500 IU CARTON
GRIFOLS
NDC 68516-3201-1
Factor IX
Complex
Profilnine® SD
Solvent Detergent Treated
5 mL
MID
PROFILNINE SD
factor ix complex
kit |
|
|
|
|
|
|
| Part 1 of 2 |
PROFILNINE SD
factor ix complex
injection, powder, lyophilized, for solution |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Part 2 of 2 |
STERILE WATER
water
injection |
|
|
|
|
|
|
|
|
|
|
|
|
|
PROFILNINE SD
factor ix complex
kit |
|
|
|
|
|
|
| Part 1 of 2 |
PROFILNINE SD
factor ix complex
injection, powder, lyophilized, for solution |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Part 2 of 2 |
STERILE WATER
water
injection |
|
|
|
|
|
|
|
|
|
|
|
|
|
PROFILNINE SD
factor ix complex
kit |
|
|
|
|
|
|
| Part 1 of 2 |
PROFILNINE SD
factor ix complex
injection, powder, lyophilized, for solution |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Part 2 of 2 |
STERILE WATER
water
injection |
|
|
|
|
|
|
|
|
|
|
|
|
|
Revised: 08/2010Grifols Biologicals Inc.