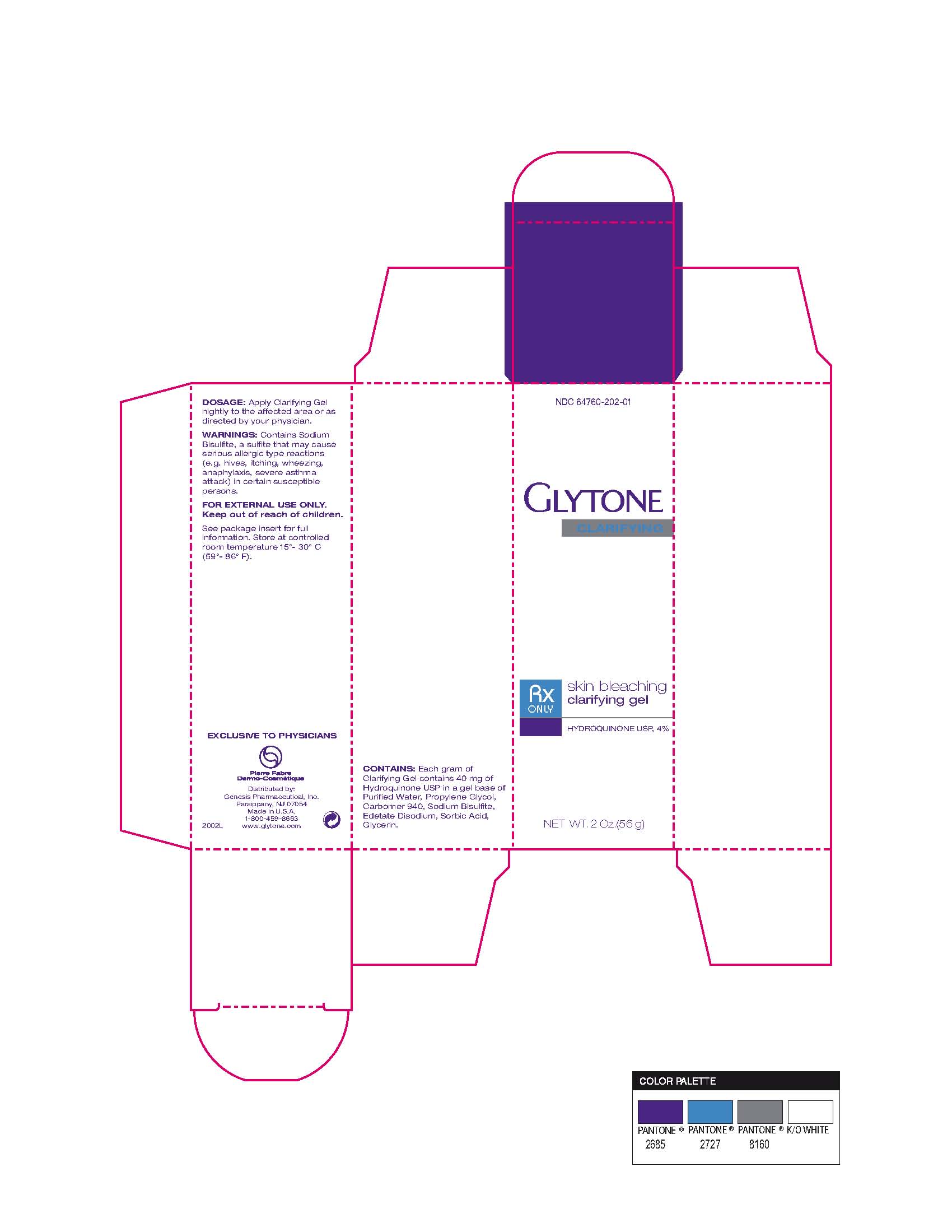
GLYTONE CLARIFYING - hydroquinone gel
Glytone
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Glytone Skin bleaching Clarifying
Warnings Contains Sodium Bisulfite, a sulfite that may cause serious allergic type reactions (eg hives, itching, wheezing, anaphlyaxis, severe asthma attack) in certain susceptible persons.
| GLYTONE CLARIFYING
hydroquinone gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Glytone (117196928) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genesis Pharmaceutical | 117196928 | manufacture | |
Revised: 3/2010
Document Id: d4366402-fd05-48e3-a16c-c4355d2b4ffc
Set id: ad96d53f-431f-439f-9948-784f467b53dc
Version: 2
Effective Time: 20100310
Glytone