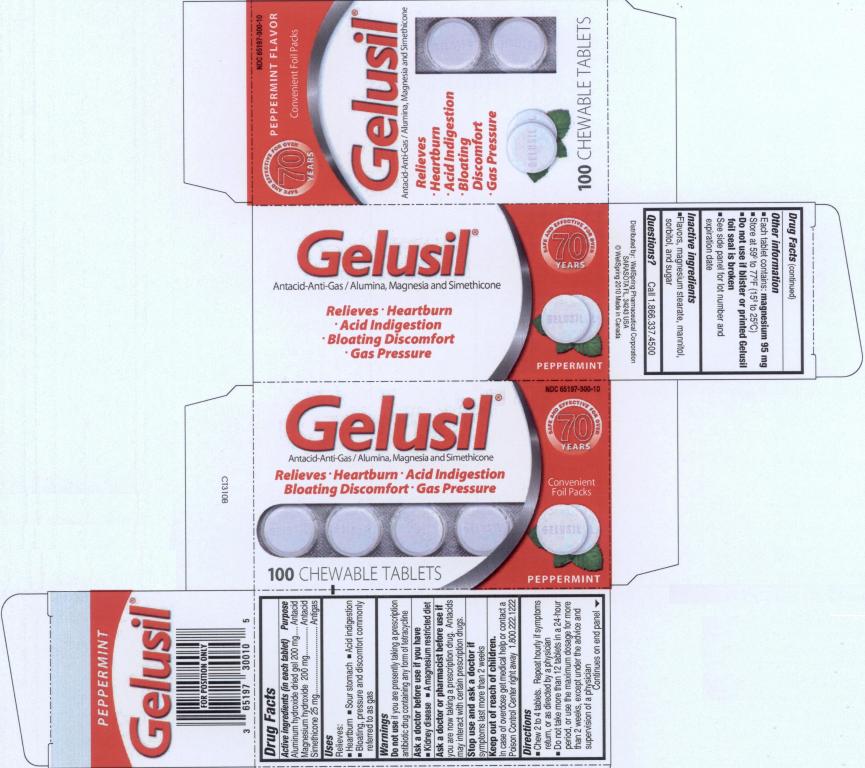
GELUSIL- aluminum hydroxide, dimethicone 410, magnesium hydroxide and silicon dioxide tablet, chewable
WellSpring Pharmaceutical Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients
Active ingredients (in each tablet)
Aluminum hydroxide dried gel 200 mg
Magnesium hydroxide 200 mg
Simethicone 25 mg
Uses
Relieves:
- heartburn
- sour stomach
- acid indigestion
- bloating, pressure and discomfort commonly referred to as gas
Warnings
Do not use if you are presently taking a prescription antibiotic drug containing any form of tetracycline
Directions
- Chew 2 to 4 tablets. Repeat hourly if symptoms return, or as directed by a physician.
- Do not take more than 12 tablets in a 24-hour period, or use the maximum dosage for more than 2 weeks, except under the advice and supervision of a physician
Other information
- each tablet contains: magnesium 95 mg
- store at 59º to 77º (15º to 25ºC)
- do not use if blister or printed Gelusil foil seal is broken
- see side panel for lot number and expiration date
| GELUSIL
aluminum hydroxide dried gel and magnesium hydroxide and simethicone tablet, chewable |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - WellSpring Pharmaceutical Corporation (110999054) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Trillium Health Care Manufacturing Inc. | 255426306 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| WellSpring Pharmaceutical Corporation | 110999054 | MANUFACTURE | |
Revised: 01/2011
Document Id: 136e1dde-f967-49cb-890a-63792904c8c6
Set id: 92fbeec1-1562-40f3-9734-41f133d494f9
Version: 7
Effective Time: 20110103
WellSpring Pharmaceutical Corporation