IMUFLEX WB-SP BLOOD BAG SYSTEM WITH INTEGRAL WHOLE BLOOD LEUKOCYTE REDUCTION FILTER (SAVING PLATELETS) WITH DIVERSION BLOOD SAMPLING ARM ANTICOAGULANT CITRATE PHOSPHATE DEXTROSE (CPD) AND OPTISOL (AS-5) RED CELL PRESERVATIVE
-
anticoagulant citrate phosphate dextrose (cpd) and as-5 red cell preservative
Terumo Corporation
----------
IMUFLEX® WB-SP BLOOD BAG SYSTEM WITH INTEGRAL WHOLE BLOOD LEUKOCYTE REDUCTION FILTER (SAVING PLATELETS) WITH DIVERSION BLOOD SAMPLING ARM® CPD/OPTISOL® SOLUTION Issued 06/06N-SP-OP-A-DB 1
IMUFLEX® WS-RP BLOOD BAG SYSTEM WITH INTEGRAL WHOLE BLOOD LEUKOCYTE REDUCTION FILTER (SAVING PLATELETS) WITH DIVERSION BLOOD SAMPLING ARM® CPD/OPTISOL® SOLUTION
Read these instructions carefully before use. Rx ONLY.
Integral filter unit intended for leukocyte reduction of Whole Blood up to 8 hours after blood collection when Whole Blood is stored at ambient temperature. Preparation of leukocyte reduced red blood cells, plasma, and platelet units may be accomplished with this set. The leukocyte reduced blood components may then be
stored for the maximum allowable dating period as indicated in the section INSTRUCTIONS FOR COMPONENT SEPARATION.
• For single use only. Sterile and non-pyrogenic fluid path. Sterilized by steam.
• Intended for the collection, processing and preservation of human blood and components.
PRECAUTIONS
• Do not use unless the solutions are clear.
• Avoid excessive heat and direct sunlight. Protect from freezing.
• Recommended storage conditions: Room Temperature (15-30°C/59-86°F).
CAUTION Do not use a dielectric tube sealer to seal the tubing while the needle is connected to the donor's body unless it is approved for such a purpose.
INSTRUCTIONS FOR BLOOD COLLECTION: Use aseptic technique
(See Fig. 1,Fig. 2a and Fig. 2b )
Materials Needed: Evacuated Blood Collection Tubes
1. Prepare the blood bag following your institution's standard operating procedures.
2. Make a loose knot in the donor tubing below the "Y" and CLIKTIP (inline closure device) unless alternate methods are used to seal the tubing at the end of collection.
3. Temporarily clamp the donor tubing between the phlebotomy needle and the "Y".
Insert Fig. 1 here
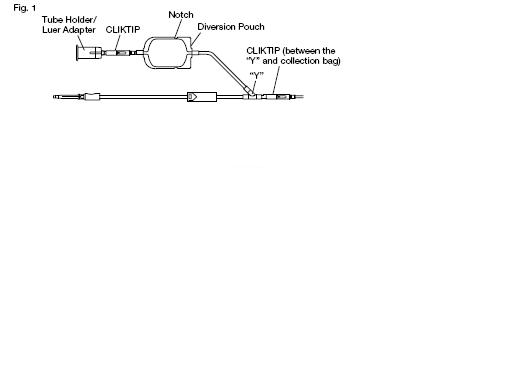
4. Suspend the collection bag as far as possible below the donor's arm.
5. Apply blood pressure cuff or tourniquet to donor's arm. Disinfect site of phlebotomy. If blood pressure cuff is used, inflate to approximately 60 mmHg.
6. Remove the needle cover and perform phlebotomy. Remove the temporary clamp on the donor tubing to permit blood flow into the Diversion Blood Sampling Arm pouch.
CAUTION Do not touch the needle after removing the needle protector.
7. Secure the needle safety device in place following the manufacturer's instructions.
8. Secure donor tubing to donor's arm.
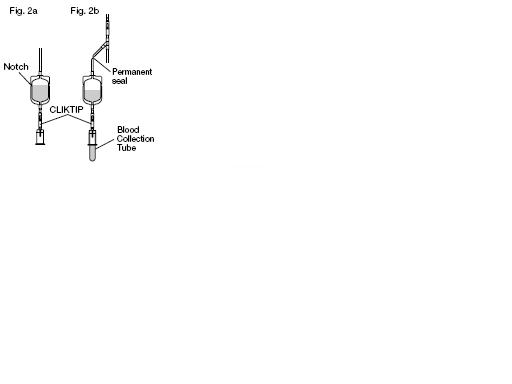
9. Position the diversion pouch with the notches up and the Tube Holder/Luer Adapter assembly down. When the level of blood in the pouch is approximately in line
with the notches, the diversion pouch is full. (Fig. 2a)
NOTE: The approximate fill volume of the pouch at the notches is 35 mL.
10. Permanently seal the tubing between the "Y" and the diversion pouch to maintain a closed system using an aluminum clip or a tube sealer approved for use with tubing connected to a donor. (Fig. 2b)
11. To initiate blood flow into the collection bag, break the CLlKTIP between the "Y" and the collection bag.
12. To avoid clot formation, collect samples as soon as possible from the diversion pouch as follows (Fig. 2b)
CAUTION Do not collect donor test samples until the tubing between the "Y" and the diversion pouch is permanently sealed.
a) Break the CLIKTIP in the tubing below the pouch to open the pathway for sampling. (Fig. 2b)
b) Position the diversion pouch with the notches up and the Tube Holder/Luer Adapter assembly downward. Assure that any air in the pouch is at the top and will not enter the
blood collection tubes.
c) Open the Tube Holder lid and insert blood collection tube firmly into the tube holder; when full, remove sample tube from holder. Repeat to collect additional samples.
NOTE: The pouch may be removed after the donor test samples are collected. A second seal must be made between the diversion pouch and the permanent seal
prior to removing the pouch.
Insert Fig. 2a and 2b here
13. Mix blood with anticoagulant in the collection bag and continue to mix at several intervals during collection and immediately after collection. If using an automated mixer, follow
manufacturer's instructions.
14. Collect labeled volume of blood (±10%).
15. When the desired amount of blood has been collected, seal the tubing or tighten the loose knot (white knot) prepared in step 2. Make a second seal between the first seal or knot and
the CLlKTIP below the "Y". Various methods may be used to seal tubing.
16. Release pressure on the donor's arm and remove the needle into the needle safety device following the manufacturer's instructions. Sever the donor tubing between the two seals
previously made below the CLlKTIP and "Y".
CAUTIONDiscard the Diversion Blood Sampling Arm and phlebotomy needle/donor tubing according to institutional procedures.
17. Seal and remove donor tubing from collection bag or strip donor tubing as follows:
a) To obtain a quality control prefiltration sample, strip blood from donor tubing into collection bag, mix well, and allow tubing to refill; repeat once. Leave an adequate length of tubing
containing the well-mixed anticoagulated whole blood attached to the collection bag.
OR
b) To maximize collection recovery, strip the tubing, mix well and seal tubing close to the collection bag without refilling. Remove tubing from collection bag.
INSTRUCTIONS FOR BLOOD FILTRATION
(See Fig. 3)
NOTE: Wait 1 hour after collection before filtering. Filtration can be accomplished when blood is stored at room temperature for up to eight hours after collection. Remove the tube guides (not depicted) from the coiled tubing prior to filtration.
Insert Fig. 3 here
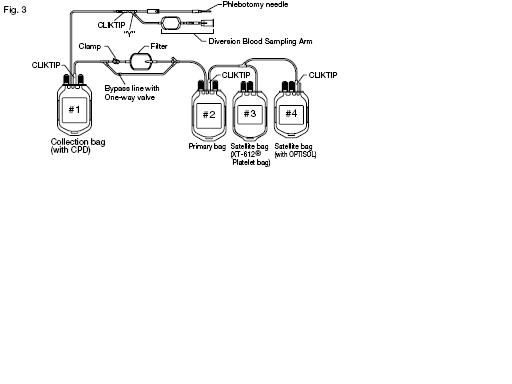
1. Mix the unfiltered whole blood unit by inverting the collection bag #1 several times.
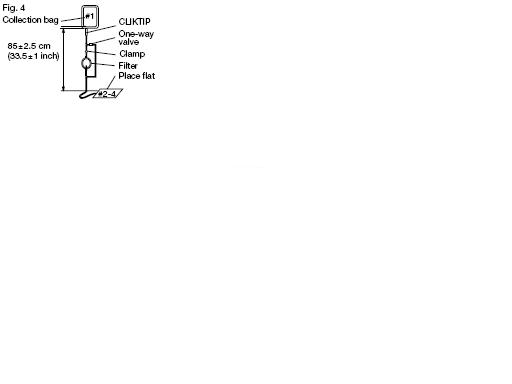
2. Hang the collection bag #1, and extend the filtration set to 85 + or - 2.5 cm or 33.5 + or - 1 inch. Verify that the filter is vertical and ensure all tubing is freely suspended. Position and support bags #2-4 on a flat surface (Fig. 4).
Note: This distance is measured from the outlet of the collection bag to the position of the supported bags as indicated in Fig. 4.
Verify tubing has been removed from the tube guides and extends freely.
Insert Fig. 4 here
3. Break the CLlKTIP at the outlet of the collection bag #1 and start filtration.
CAUTIONDo not squeeze the collection bag during filtration. Do not squeeze or apply pressure on the filter while it is attached to the bag containing the filtered blood. If for any reason whole blood passes through the one-way valve, stop filtration immediately and consider the product as non-leukocyte reduced.
4. Filtration ends when the collection bag is empty or blood flow has stopped. Close the WHITE clamp above the filter.
5. Expel air through the bypass line and back into the empty collection bag #1 by holding the primary bag #2 upright so that the air will be closest to the ports. Gently squeeze the primary bag containing the leukoreduced whole blood until filtered blood reaches the "Y" below the filter.
6. Open the WHITE clamp.
7. Recovery of filtered Whole Blood is complete when the collection bag and the inlet side of the filter have drained or blood flow has stopped. Close the WHITE clamp.
8. Seal the tubing as close as possible to the "Y" below the filter and properly dispose of the filter and collection bag #1.
9. Strip the post filter tubing into the primary bag #2, mix well, and allow tubing to refill; repeat once. Make an appropriate number of segments necessary for testing by sealing on or near
the X marks. Leave segments attached to the filtered whole blood unit.
INSTRUCTIONS FOR COMPONENT SEPARATION
NOTE:
• Platelets should be separated from the Red Blood Cells within 8 hours of blood collection.
• Fresh Frozen Plasma should be separated from the Red Blood Cells and placed in a freezer at -18°C or colder within 8 hours of collection.
• OPTISOL should be added to the Red Blood Cells immediately after removal of the plasma. If plasma is not separated from the Red Blood Cells within 8 hours, OPTISOL
may be added within 72 hours of collection if Whole Blood is refrigerated.
• Other plasma components may be prepared following approved regulations and standards.
1. Follow your institution's standard component processing procedures to prepare components.
2. Centrifuge flltered whole blood unit to separate red blood cells from platelet rich plasma.
3. Break the CLlKTIP of primary bag #2 and transfer leukocytes-reduced platelet rich plasma into XT-612 Platelet bag #3. Clamp the transfer tubing on the Platelet bag.
4. Break the CLlKT1P of OPTlSOL Solution bag #4 and drain contents into primary bag #2 containing red blood cells. Seal tubing of primary bag in two places, and cut between seals
and separate from Platelet and OPTISOL bags.
NOTE: Empty OPTISOL bag is used for further component preparation.
5. Invert the red blood cell- OPTISOL mixture several times to insure the final product is well suspended.
6. Store AS-5 Red Blood Cells, Leukocytes Reduced between 1-6°C for up to 42 days.
NOTE: Whole Blood or Red Blood Cells in CPD may be stored for up to 21 days at 1-6°C.
Store Platelets, Leukocytes Reduced between 20-24°C for up to 5 days
BLISTER PACKAGE
(See Fig. 5)
To open blister package, peel the cover film back four fifths of its length. After opening, the blood bag system may be stored at room temperature for 7 days, or it may be stored for for 30 days in the blister package after returning the cover film to the original position and
sealing with tape to prevent possible loss of moisture.
Insert Fig. 5 here

CAUTIONS
•The AGELESS packet contained in this package absorbs oxygen and generates heat on removal. Do not open and handle it with care.
• Dispose of the AGELESS packet with the blister tray.
• Do not dispose with wastes containing volatile or flammable materials.
TERUMO® MANUFACTURED BY:TERUMO CORPORATION44-1, 2-CHOME, HATAGAYA, SHIBUYA-KU, TOKYO, JAPAN MADE IN JAPAN
®: Registered Trademark © TERUMO CORPORATION 2006 06F20
Tray/Case Label
BLOOD LEUKOCYTE REDUCTION FILTER (SAVING PLATELETS) WITH DIVERSION
BLOOD SAMPLING ARM®
CPD WITH OPTISOL® RED CELL PRESERVATIVE SOLUTION
FOR COLLECTION OF 500mL OF BLOOD
Each unit consists of a collection bag containing 70mL of Anticoagulant
CPD solution, with a satellite bag containing 111mL of OPTlSOL Red
Cell Preservative Solution.
Each 70mL Anticoagulant CPD solution USP contains 1.79g Dextrose
(monohydrate) USP, 1.84g Sodium Citrate (dihydrate) USP, 209mg Citric
Acid (anhydrous) USP, 156mg Monobasic Sodium Phosphate
(monohydrate) USP.
Each 111mL OPTISOL Red Cell Preservative Solution contains 974mg
Sodium Chloride USP, 1.00g Dextrose (monohydrate) USP, 583mg
Mannitol USP, 33.3mg Adenine USP.
STERILE, NON-PYROGENIC FLUID PATH.
DO NOT USE UNLESS SOLUTIONS ARE CLEAR.
CODE
LOT No.
EXPIRY
UNIT(S)
DONOR NEEDLE 16G x 1 1/2˝ (1.60 x 38mm)
Rx ONLY
RECOMMENDED STORAGE: Room Temperature (15-30°C/59-86°F).
Avoid excessive heat. Protect from freezing.
After opening, the blood bag system may be stored at room temperature for 7 days, or it may be stored for 30 days in the blister package after returning the cover film to the original
position and sealing with tape to prevent possible loss of moisture.
See Instructions For Use.
Manufactured by : TERUMO CORPORATION Tokyo, Japan
® : Registered Trademark
Issued 01/06
B-4-HS6-A2 1
Place Label here

| IMUFLEX WB-SP BLOOD BAG SYSTEM WITH INTEGRAL WHOLE BLOOD LEUKOCYTE REDUCTION FILTER (SAVING PLATELETS) WITH DIVERSION BLOOD SAMPLING ARM ANTICOAGULANT CITRATE PHOSPHATE DEXTROSE (CPD) AND OPTISOL (AS-5) RED CELL PRESERVATIVE
anticoagulant citrate phosphate dextrose (cpd) and as-5 red cell preservative kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA880217 | 12/15/2010 | |
| Labeler - Terumo Corporation (690543319) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Terumo Corp. - Fujinomiya Factory | 695214015 | manufacture | |