gentamicin sulfate (Gentamicin Sulfate) injection, solution
[Baxter Healthcare Corporation]
in Plastic Container
VIAFLEX Plus Container
For Intravenous Administration
Further Dilution Not Required
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Isotonic Gentamicin Sulfate Injection and other antibacterial drugs, Isotonic Gentamicin Sulfate Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
WARNINGS
Patients treated with aminoglycosides should be under close clinical observation because of the potential toxicity associated with their use.
As with other aminoglycosides, gentamicin sulfate is potentially nephrotoxic. The risk of nephrotoxicity is greater in patients with impaired renal function and in those who receive high dosage of prolonged therapy.
Neurotoxicity manifested by ototoxicity, both vestibular and auditory, can occur in patients treated with gentamicin sulfate, primarily those with preexisting renal damage and in patients with normal renal functions treated with higher doses or for longer periods than recommended. Aminoglycoside-induced ototoxicity is usually irreversible. Other manifestations of neurotoxicity may include numbness, skin tingling, muscle twitching, and convulsions.
Renal and eighth cranial nerve function should be closely monitored, especially in patients with known or suspected reduced renal function at onset of therapy and also in those whose renal function is initially normal but who develop signs of renal dysfunction during therapy. Urine should be examined for decreased specific gravity, increased excretion of protein, and the presence of cells or casts. Blood urea nitrogen, serum creatinine, or creatinine clearance should be determined periodically. When feasible, it is recommended that serial audiograms be obtained in patients old enough to be tested, particularly high risk patients. Evidence of ototoxicity (dizziness, vertigo, tinnitus, roaring in the ears or hearing loss) or nephrotoxicity requires dosage adjustment or discontinuance of the drug. As with the other aminoglycosides, on rare occasions changes in renal and eighth cranial nerve function may not become manifest until soon after completion of therapy.
Serum concentrations of aminoglycosides should be monitored, when feasible, to assure adequate levels and to avoid potentially toxic levels. When monitoring gentamicin peak concentrations, dosage should be adjusted so that prolonged levels above 12 mcg/mL are avoided. When monitoring gentamicin trough concentrations, dosage should be adjusted so that levels above 2 mcg/mL are avoided.
Excessive peaks and/or trough serum concentrations of aminoglycosides may increase the risk of renal and eighth cranial nerve toxicity. In the event of overdosage or toxic reactions, hemodialysis may aid in the removal of gentamicin from the blood, especially if renal function is, or becomes, compromised. The rate of removal of gentamicin is considerably lower by peritoneal dialysis than it is by hemodialysis.
Concurrent and/or sequential systemic or topical use of other potentially neurotoxic and/or nephrotoxic drugs, such as cisplatin, cephaloridine, kanamycin, amikacin, neomycin, polymyxin B, colistin, paromomycin, streptomycin, tobramycin, vancomycin, and viomycin should be avoided. Other factors which may increase patient risk of toxicity are advanced age and dehydration.
The concurrent use of gentamicin with potent diuretics, such as ethacrynic acid or furosemide, should be avoided, since certain diuretics by themselves may cause ototoxicity. In addition, when administered intravenously, diuretics may enhance aminoglycoside toxicity by altering the antibiotic concentration in serum and tissue.
Aminoglycosides can cause fetal harm when administered to a pregnant woman (see WARNINGS section).
DESCRIPTION
Gentamicin Sulfate, USP, a water soluble antibiotic of the aminoglycoside group, is derived from Micromonospora purpurea, and actinomycete.
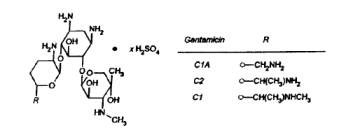
Isotonic Gentamicin Sulfate Injection is a sterile, nonpyrogenic solution of Gentamicin Sulfate, USP in water for injection with 9 mg/mL sodium chloride (NaCl) to provide isotonicity. The solution is intended for intravenous use and requires no further dilution. pH may be adjusted with sulfuric acid or sodium hydroxide and is approximately 4.5.
This VIAFLEX Plus plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). VIAFLEX Plus on the container indicates the presence of a drug additive in a drug vehicle. The VIAFLEX Plus plastic container system utilizes the same container as the VIAFLEX plastic container system. The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million. However, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies.
CLINICAL PHARMACOLOGY
When gentamicin is administered by intravenous infusion over a two hour period, peak serum concentrations usually occur between 30 and 60 minutes and serum levels are measurable for six to eight hours.
In patients with normal renal function, peak serum concentrations of gentamicin (mcg/mL) are usually up to four times the single intramuscular dose (mg/kg); for example, a 1 mg/kg injection in adults may be expected to result in a peak serum concentration up to 4 mcg/mL, a 1.5 mg/kg dose may produce levels up to 6 mcg/mL. While some variation is to be expected due to a number of variables such as age, body temperature, surface area and physiologic differences, the individual patient given the same dose tends to have similar levels in repeated determinations. Gentamicin administered at 1 mg/kg every eight hours for the usual 7 to 10 day treatment period to patients with normal renal function does not accumulate in the serum.
Gentamicin, like all aminoglycosides, may accumulate in the serum and tissue of patients treated with higher doses and for prolonged periods, particularly in the presence of impaired renal function. In adult patients, treatment with a gentamicin dose of 4 mg/kg/day or higher over a seven to ten day treatment period may result in a slight, progressive rise in both peak and trough concentrations. In patients with impaired renal function, serum concentrations of gentamicin are usually higher and measurable for longer periods. The more severe the impairment, the slower the clearance. (Dosage must be adjusted.)
Since gentamicin is distributed in extracellular fluid, peak serum concentrations may be lower than usual in adult patients who have a large volume of this fluid. Serum concentrations of gentamicin in febrile patients may be lower than those in afebrile patients given the same dose. When body temperature returns to normal, serum concentrations of the drug may rise. Febrile and anemic states may be associated with a shorter than usual serum half-life. (Dosage adjustment is usually not necessary.) In severely burned patients the half-life may be significantly decreased, and resulting serum concentrations may be lower than anticipated from the mg/kg dose. Protein binding studies have indicated that the degree of gentamicin binding is low; depending upon the methods used for testing, this may be between 0 and 30%.
After initial administration to patients with normal renal function, generally 70% or more of the gentamicin dose is recoverable in the urine in 24 hours; concentrations in urine above 100 mcg/mL may be achieved. Little, if any, metabolic transformation occurs; the drug is excreted principally by glomerular filtration. After several days of treatment, the amount of gentamicin excreted in the urine approaches the daily dose administered. As with other aminoglycosides, a small amount of the gentamicin dose may be retained in the tissues, especially in the kidneys. Minute quantities of gentamicin have been detected in the urine weeks after the drug administration was discontinued. Renal clearance of gentamicin is similar to that of endogenous creatinine.
In patients with marked impairment of renal function, there is a decrease in the concentration of aminoglycosides in urine and in their penetration into defective renal parenchyma. This decreased drug excretion, together with the potential nephrotoxicity of aminoglycosides, should be considered when treating patients with urinary tract infections.
Probenecid does not affect renal tubular transport of gentamicin.
Endogenous creatinine clearance rate and serum creatinine level have a high correlation with the half-life of gentamicin in serum. Results of these tests may serve as guides for adjusting dosage in patients with renal impairment (and DOSAGE AND ADMINISTRATION).
Following parenteral administration, gentamicin can be detected in serum, lymph, tissues, sputum, and in pleural, synovial, and peritoneal fluids. Concentrations in renal cortex sometimes may be eight times higher than the usual serum levels. Concentrations in bile, in general, have been low and have suggested minimal biliary excretion. Gentamicin crosses the peritoneal as well as the placental membranes. Since aminoglycosides diffuse poorly into the subarachnoid space after parenteral administration, concentrations of gentamicin in cerebrospinal fluid are often low and dependent upon dose, rate of penetration, and degree of meningeal inflammation. There is minimal penetration of gentamicin into ocular tissues following intravenous administration.
Microbiology:In vitro tests have demonstrated that gentamicin is a bactericidal antibiotic which acts by inhibiting normal protein synthesis in susceptible microorganisms. It is active against a wide variety of pathogenic bacteria including Escherichia coli, Proteus sp. (indole-positive and indole-negative), Pseudomonas aeruginosa, species of the Klebsiella- Enterobacter-Serratia group, Citrobacter sp. and Staphylococcus sp. (including penicillin and methicillin-resistant strains). Gentamicin is also activein vitro against species ofSalmonella and Shigella. The following bacteria areusually resistant to aminoglycosides: Streptococcus pneumoniae, most species of Streptococci, particularly group D and anaerobic organisms, such as Bacteriods sp. or Clostridium sp.
In vitro studies have shown that an aminoglycoside combined with an antibiotic that interferes with cell wall synthesis may act synergistically against some group D streptococcal strains. The combination of gentamicin and penicillin G has a synergistic bactericidal effect against virtually all strains ofStreptococcus faecalis and its varieties (S faecalis var.liquifaciens, S faecalis var. zymogenes), S faecium and S durans. An enhanced killing effect against many of these strains has also been shown in vitro with combinations of gentamicin and ampicillin, carbenicillin, nafcillin, or oxacillin.
The combined effect of gentamicin and carbenicillin is synergistic for many strains ofPseudomonas aeruginosa.In vitro synergism against other gram-negative organisms has been shown with combinations of gentamicin and cephalosporins.
Gentamicin may be active against clinical isolates of bacteria resistant to other aminoglycosides. Bacteria resistant to one aminoglycoside may be resistant to one or more other aminoglycosides. Bacterial resistance to gentamicin is generally developed slowly.
Susceptibility Testing: If the disc method of susceptibility testing used is that described by Bauer et al (Am J Clin Path 45:493, 1966; Federal Register 37:20527-20529, 1972), a disc containing 10 mcg of gentamicin should be given a zone of inhibition of 15 mm or more to indicate susceptibility of the infecting organism. Zones greater than 12 mm and less than 15 mm indicate intermediate susceptibility. A zone of 12 mm or less indicates that the infecting organism is likely to be resistant. In certain conditions it may be desirable to do additional susceptibility testing by the tube or agar dilution method; gentamicin substance is available for this purpose.
INDICATIONS AND USAGE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Isotonic Gentamicin Sulfate Injection and other antibacterial drugs, Isotonic Gentamicin Sulfate Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Isotonic Gentamicin Sulfate Injection is indicated in the treatment of serious infections caused by susceptible strains of the following microorganisms: Pseudomonas aeruginosa, Proteus sp. (indole-positive and indole-negative), Escherichia coli, Klebsiella-Enterobacter- Serratia sp., Citrobacter sp., and Staphylococcus sp. (coagulase-positive and coagulase-negative).
Clinical studies have shown gentamicin sulfate to be effective in bacterial neonatal sepsis: bacterial septicemia, and serious bacterial infections of the central nervous system (meningitis), urinary tract, respiratory tract, gastrointestinal tract (including peritonitis), skin, bone and soft tissue (including burns).
Aminoglycosides, including gentamicin, are not indicated in uncomplicated initial episodes of urinary tract infections, unless the causative organisms are susceptible to these antibiotics, and are not susceptible to antibiotics having less potential for toxicity.
Specimens for bacterial culture should be obtained to isolate and identify causative organisms, and to determine their susceptibility to gentamicin.
Isotonic Gentamicin Sulfate Injection may be considered as initial therapy in suspected or confirmed gram-negative infections, and therapy may be instituted before obtaining results of susceptibility testing. The decision to continue therapy with this drug should be based on the results of susceptibility tests, the severity of the infection, and the important additional concepts contained in the WARNINGSBox above. If the causative organisms are resistant to gentamicin, other appropriate therapy should be instituted.
In serious infections when the causative organisms are unknown, Isotonic Gentamicin Sulfate Injection may be administered as initial therapy in conjunction with a penicillin or cephalosporin type drug before obtaining results of susceptibility testing. If anaerobic organisms are suspected as etiologic agents, consideration should be given to using other suitable antimicrobial therapy in conjunction with gentamicin. Following identification of the organism and its susceptibility, appropriate antibiotic therapy should then be continued.
Gentamicin has been used effectively in combination with carbenicillin for the treatment of life-threatening infections caused by Pseudomonas aeruginosa. It has also been found effective when used in conjunction with a penicillin type drug for the treatment of endocarditis caused by group D streptococci.
Gentamicin sulfate has also been shown to be effective in the treatment of serious staphylococcal infections. While not the antibiotic of first choice, gentamicin sulfate may be considered when penicillins or other less potentially toxic drugs are contraindicated and bacterial susceptibility tests and clinical judgement indicate its use. It may also be considered in mixed infections caused by susceptible strains of staphylococci and gram-negative organisms.
In the neonate with suspected sepsis or staphylococcal pneumonia, a penicillin type drug is also usually indicated as concomitant therapy with gentamicin.
CONTRAINDICATIONS
Hypersensitivity to gentamicin is a contraindication to its use. A history of hypersensitivity or serious toxic reactions to aminoglycosides may also contraindicate use of gentamicin because of known cross-sensitivity of patients to drugs in this class.
WARNINGS
Warnings: (See Box WARNING) Aminoglycosides can cause fetal harm when administered to a pregnant woman. Aminoglycoside antibiotics cross the placenta, and there have been several reports of total irreversible bilateral congenital deafness in children whose mothers received streptomycin during pregnancy. Animal reproduction studies conducted on rats and rabbits did not reveal evidence of impaired fertility or harm to the fetus due to gentamicin sulfate. It is also not known whether gentamicin sulfate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Serious side effects to mother, fetus, or newborn have not been reported in the treatment of pregnant women with other aminoglycosides. If gentamicin is used during pregnancy or if the patient becomes pregnant while taking gentamicin, she should be apprised of the potential hazard to the fetus.
Solutions containing sodium ion should be used with great care in patients with congestive heart failure, severe renal insufficiency, and in clinical states in which there exists edema with sodium retention.
In patients with diminished renal function, administration of solutions containing sodium ions may result in sodium retention.
PRECAUTIONS
General - Prescribing Isotonic Gentamicin Sulfate Injection in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Do not use additives or premix with other drugs. (see DOSAGE AND ADMINSTRATION).
Neurotoxic and nephrotoxic antibiotics may be almost completely absorbed from body surfaces (except the urinary bladder) after local irrigation and after topical application during surgical procedures. The potential toxic effects of antibiotics administered in this fashion (neuromuscular blockade, respiratory paralysis, oto-and nephrotoxicity) should be considered (see WARNINGS box).
Increased nephrotoxicity has been reported following concomitant administration of aminoglycoside antibiotics and cephalosporins.
Neuromuscular blockage and respiratory paralysis have been reported in the cat receiving high doses (40 mg/kg) of gentamicin. The possibility of these phenomena occurring in man should be considered if aminoglycosides are administered by any route to patients receiving anesthetics or to patients receiving neuromuscular blocking agents, such as succinylcholine, tubocurarine, or decamethonium, or in patients receiving massive transfusions of citrate-anticoagulated blood. If neuromuscular blockade occurs, calcium salts may reverse it.
Aminoglycosides should be used with caution in patients with neuromuscular disorders such as myasthenia gravis or parkinsonism, since these drugs may aggravate muscle weakness because of their potential curare-like effects on the neuromuscular junction. During or following gentamicin therapy, paresthesias, tetany, positive, Chvostek and Trousseau signs and mental confusion have been described in patients with hypomagnesemia, hypocalcemia and hypokalemia. When this has occurred in infants, tetany and muscle weakness has been described. Both adults and infants required appropriate corrective electrolyte therapy.
Elderly patients may have reduced renal function which may not be evident in the results of routine screening tests such as BUN or serum creatinine. A creatinine clearance determination may be more useful. Monitoring of renal function during treatment with gentamicin, as with other aminoglycosides, is particularly important in such patients. A Fanconi-like syndrome, with aminoaciduria and metabolic acidosis has been reported in some adults and infants being given gentamicin injections.
Cross allergenicity among aminoglycosides has been demonstrated.
Patients should be well hydrated during treatment.
Although thein vitro mixing of gentamicin and carbenicillin results in a rapid and significant inactivation of gentamicin, this interaction has not been demonstrated in patients with normal renal function who received both drugs by different routes of administration. A reduction in gentamicin serum half-life has been reported in patients with severe renal impairment receiving carbenicillin concomitantly with gentamicin.
Treatment with gentamicin may result in overgrowth of nonsusceptible organisms. If this occurs, appropriate therapy is indicated.
See WARNINGS Box regarding concurrent use of potent diuretics and regarding concurrent and/or sequential use of other neurotoxic and/or nephrotoxic antibiotics, and for other essential information.
Caution must be exercised in the administration of parenteral fluids, especially those containing sodium ion, to patients receiving corticosteroids or corticotropin.
Pregnancy Category D
(see WARNINGS section)
Do not administer unless solution is clear and seal is intact.
Information for Patients
Patients should be counseled that antibacterial drugs including Isotonic Gentamicin Sulfate Injection, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Isotonic Gentamicin Sulfate Injection is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Isotonic Gentamicin Sulfate Injection or other antibacterial drugs in the future.
ADVERSE REACTIONS
Nephrotoxicity - Adverse renal effects, as demonstrated by the presence of casts, cells, or protein in the urine or by rising BUN, NPN, serum creatinine and oliguria, have been reported. They occur more frequently in patients with a history of renal impairment and in patients treated for longer periods or with larger dosages than recommended.
Neurotoxicity - Serious adverse effects on both vestibular and auditory branches of the eighth nerve have been reported, primarily in patients with renal impairment (especially if dialysis is required) and in patients on high doses and/or prolonged therapy. Symptoms include dizziness, vertigo, tinnitus, roaring in the ears and hearing loss, which, as with the other aminoglycosides, may be irreversible. Hearing loss is usually manifested initially by diminution of high-tone acuity. Other factors which may increase the risk of toxicity include excessive dosage, dehydration and previous exposure to other ototoxic drugs.
Peripheral neuropathy or encephalopathy, including numbness, skin tingling, muscle twitching, convulsions, and a myasthenia gravis-like syndrome, have been reported.
Note: This risk of toxic reactions is low in patients with normal renal function who do not receive gentamicin sulfate at higher doses, or for longer periods of time than recommended.
Other reported adverse reactions possibly related to gentamicin include: respiratory depression, lethargy, confusion, depression, visual disturbances, decreased appetite, weight loss, hypotension and hypertension; rash, itching, urticaria, generalized burning, laryngeal edema, anaphylactoid reactions, fever, and headache; nausea, vomiting, increased salivation, and stomatitis; purpura, pseudotumor cerebri, acute organic brain syndrome, pulmonary fibrosis, alopecia, joint pain, transient hepatomegaly, and splenomegaly.
Laboratory abnormalities possibly related to gentamicin include: increased serum transaminase (SGOT, SGPT), serum LDH and bilirubin; decreased serum calcium, magnesium, sodium and potassium; anemia, leukopenia, granulocytopenia, transient agranulocytosis, eosinophilia, increased and decreased reticulocyte counts, and thrombocytopenia. While clinical laboratory test abnormalities may be isolated findings, they may also be associated with clinically related signs and symptoms. For example, tetany and muscle weakness may be associated with hypomagnesemia, hypocalcemia, and hypokalemia.
While local tolerance of gentamicin sulfate is generally excellent, there has been an occasional report of pain at the injection site. Subcutaneous atrophy or fat necrosis suggesting local irritation has been rarely reported.
Reactions which may occur because of the solution or the technique of administration, include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation, and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures, and save the remainder of the fluid for examination if deemed necessary.
OVERDOSAGE
In the event of overdosage or toxic reactions, hemodialysis may aid in the removal of gentamicin from the blood, and is especially important if renal function is, or becomes compromised. The rate of removal of gentamicin is considerably lower by peritoneal dialysis than it is by hemodialysis.
DOSAGE AND ADMINISTRATION
It is desirable to limit the duration of treatment with aminoglycosides to short term.
The usual duration of treatment for all patients is seven to ten days. In difficult and complicated infections, a longer course of therapy may be necessary. In such cases monitoring of renal, auditory, and vestibular functions is recommended, since toxicity is more apt to occur with treatment extended for more than ten days. Dosage should be reduced if clinically indicated.
The patient’s pretreatment body weight should be obtained for calculation of correct dosage. The dosage of aminoglycosides in obese patients should be based on an estimate of the lean body mass.
In patients with extensive burns, altered pharmacokinetics may result in reduced serum concentrations of aminoglycosides. In such patients treated with gentamicin, measurement of serum concentrations is recommended as a basis for dosage adjustment.
Generally, the peak concentration is expected to be in the range of 4 to 6 mcg/mL. When monitoring peak concentrations (at 30 to 60 minutes after injection), prolonged levels above 12 mcg/mL should be avoided. When monitoring trough concentrations (just prior to the next dose), levels above 2 mcg/mL should be avoided. Determination of the adequacy of serum level for a particular patient must take into consideration the susceptibility of the causative organism, the severity of the infection, and the status of the patient’s host defense mechanisms.
The intravenous administration of gentamicin may be particularly useful for treating patients with bacterial septicemia or those in shock. It may also be the preferred route of administration for some patients with congestive heart failure, hematologic disorders, severe burns, or those with reduced muscle mass. The solution may be infused over a period of one half to two hours.
Isotonic Gentamicin Sulfate Injection should not be physically premixed with other drugs, but should be administered separately in accordance with the recommended route of administration and dosage schedule.
The dosage recommendations which follow are not intended as rigid schedules, but are provided as guides for initial therapy, or when the measurement of gentamicin serum levels during therapy is not feasible.
Patients with Normal Renal Function
Adults: The recommended dosage of Isotonic Gentamicin Sulfate Injection for patients with serious infections and normal renal function is 3 mg/kg/day, administered in three equal doses every eight hours (Table I).
| Table I. Dosage Schedule Guide (1 mg/kg) *For Serious Infections for Adults With Normal Renal Function (Dosage at Eight Hour Intervals)† |
|||||
| Dosage Schedule | Container Selection and Adjustment Guide | ||||
| Patient’s weight‡ | Dosage
q8h
mg dose | Volume to be withdrawn from container | |||
| kg | (lb) | Container content
mg | 100 mL container
mL | 50 mL container
mL |
|
| 30 | (66) | 30 | 40 | 25 | 13 |
| 35 | (77) | 35 | 40 | 13 | 6 |
| 40 | (88) | 40 | 40 | 0 | 0 |
| 45 | (99) | 45 | 60 | 25 | 13 |
| 50 | (110) | 50 | 60 | 17 | 8 |
| 55 | (121) | 55 | 60 | 8 | 4 |
| 60 | (132) | 60 | 60 | 0 | 0 |
| 65 | (143) | 65 | 80 | 19 | 9 |
| 70 | (154) | 70 | 80 | 13 | 6 |
| 75 | (165) | 75 | 80 | 6 | 3 |
| 80 | (176) | 80 | 80 | 0 | 0 |
| 85 | (187) | 85 | 100 | 15 | 8 |
| 90 | (198) | 90 | 100 | 10 | 5 |
| 95 | (209) | 95 | 100 | 5 | 3 |
| 100 | (220) | 100 | 100 | 0 | 0 |
For patients with life-threatening infections, dosages up to 5 mg/kg/day may be administered in three or four equal doses. This dosage should be reduced to 3 mg/kg/day as soon as clinically indicated (Table II).
| Table II. Dosage Schedule Guide (1.7 mg/kg) *For Life-Threatening Infections for Adults With Normal Renal Function (Dosage at Eight Hour Intervals)† |
|||||
|
|||||
| Dosage Schedule | Container Selection and Adjustment Guide | ||||
| Patient’s weight‡ | Dosage q8hmg
dose | Volume to be withdrawn from container | |||
| kg | (lb) | Container content
mg | 100 mL container
mL | 50 mL container
mL |
|
| 30 | (66) | 51 | 60 | 15 | 8 |
| 35 | (77) | 60 | 60 | 0 | 0 |
| 40 | (88) | 66 | 80 | 18 | 9 |
| 45 | (99) | 75 | 80 | 6 | 3 |
| 50 | (110) | 83 | 100 | 17 | 9 |
| 55 | (121) | 91 | 100 | 9 | 5 |
| 60 | (132) | 100 | 100 | 0 | 0 |
| 65 | (143) | 108 | 120 | 10 | 5 |
| 70 | (154) | 116 | 120 | 3 | 2 |
| 75 | (165) | 125§ | |||
This container system may be inappropriate for the dosage requirements of children, infants, and neonates. Other dosage forms may be more appropriate.
Patients with Impaired Renal Function
Dosage must be adjusted in patients with impaired renal function. Whenever possible, serum concentrations of gentamicin should be monitored. One method of dosage adjustment is to increase the interval between administration of the usual doses. Since the serum creatinine concentration has a high correlation with the serum half-life of gentamicin, this laboratory test may provide guidance for adjustment of the interval between doses. The interval between doses (in hours) may be approximated by multiplying the serum creatinine level (mg/100 mL) by 8. For example, a patient weighing 60 kg with a serum creatinine level of 2 mg/100 mL could be given 60 mg (1 mg/kg) every 16 hours (2 x 8).
In patients with serious systemic infections and renal impairment, it may be desirable to administer the antibiotic more frequently but in reduced dosage. In such patients, serum concentrations of gentamicin should be measured so that adequate but not excessive levels result. A peak and trough concentration measured intermittently during therapy will provide optimal guidance for adjusting dosage. After the usual initial dose a rough guide for determining reduced dosage at eight hour intervals is to divide the normally recommended dose by the serum creatinine level (Table III). For example, after an initial dose of 60 mg (1 mg/kg), a patient weighing 60 kg with a serum creatinine level of 2 mg/100 mL should receive 30 mg (60 ÷ 2). It should be noted that the status of renal function may be changing over the course of the infectious process.
It is important to recognize that deteriorating renal function may require a greater reduction in dosage than that specified in the above guidelines for patients with stable renal impairment.
| Table III. Dosage Schedule Guide For Patients With Renal Impairment. (Dosage at Eight Hour Intervals after the initial dose) |
||
| Serum Creatinine (mg%) | Approximate Creatinine Clearance Rate (mL/min/1.73M²) | Percent of usual dose Shown in Table I |
| <1.0 | >100 | 100 |
| 1.1-1.3 | 71-100 | 80 |
| 1.4-1.6 | 56-70 | 65 |
| 1.7-1.9 | 46-55 | 55 |
| 2.0-2.2 | 41-45 | 50 |
| 2.3-2.5 | 36-40 | 40 |
| 2.6-3.0 | 31-35 | 35 |
| 3.1-3.5 | 26-30 | 30 |
| 3.6-4.0 | 21-25 | 25 |
| 4.1-5.1 | 16-20 | 20 |
| 5.2-6.6 | 11-15 | 15 |
| 6.7-8.0 | ≤10 | 10 |
In adults with renal failure undergoing hemodialysis, the amount of gentamicin removed from the blood may vary depending upon several factors including the dialysis method used. An eight hour hemodialysis may reduce serum concentrations of gentamicin by approximately 50%. The recommended dosage at the end of each dialysis period is 1 to 1.7 mg/kg depending upon the severity of infection. In children, a dose of 2 mg/kg may be administered.
A variety of methods are available to measure gentamicin concentrations in body fluids; these include microbiologic, enzymatic and radioimmunoassay techniques.
Instructions for the Administration of Isotonic Gentamicin Sulfate Injection.
This product is intended for use only as an IV secondary medication unit.
Use aseptic technique when removing contents from these units.
Do not add other drugs to Isotonic Gentamicin Sulfate Injection.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Use of a final filter is recommended during administration of all parenteral solutions, where possible.
All injections in VIAFLEX Plus plastic containers are intended for intravenous administration using sterile equipment.
HOW SUPPLIED
Isotonic Gentamicin Sulfate Injection in VIAFLEX Plus plastic container is available in the following sizes and concentrations.
| 50 mL units | 100 mL units | |
| Gentamicin 40 mg |
2B0850 NDC 0338-0503-41 |
2B0860 NDC 0338-0500-48 |
| Gentamicin 60 mg |
2B0851 NDC 0338-0507-41 |
2B0861 NDC 0338-0501-48 |
| Gentamicin 80 mg |
2B0852 NDC 0338-0509-41 |
2B0862 NDC 0338-0503-48 |
| Gentamicin 100 mg |
2B0853 NDC 0338-0511-41 |
2B0863 NDC 0338-0505-48 |
| Gentamicin 120 mg | 2B0854 NDC 0338-0513-41 | 2B0864 NDC 0338-0507-48 |
Do not remove unit from overwrap until ready for use. The overwrap is a moisture barrier. The inner bag maintains the sterility of the product. After removing overwrap, check for minute leaks by squeezing inner bag firmly. If leaks are found, discard solution as sterility may be impaired.
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (25°C); brief exposure up to 40°C does not adversely affect the product.
Directions for Use of VIAFLEX Plus Plastic Container
Warning: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed.
To Open
Tear overwrap down side at slit and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for leaks. Do not add supplementary medication.
Preparation for Administration
1. Suspend container from eyelet support.
2. Remove protector from outlet port at bottom of container.
3. Attach administration set. Refer to complete directions accompanying set.
Baxter, VIAFLEX and PL 146 are trademarks of Baxter International Inc.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Printed in USA
07-19-48-344
Rev. November 2006
| Gentamicin Sulfate (Gentamicin Sulfate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Gentamicin Sulfate (Gentamicin Sulfate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Gentamicin Sulfate (Gentamicin Sulfate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Gentamicin Sulfate (Gentamicin Sulfate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Gentamicin Sulfate (Gentamicin Sulfate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Gentamicin Sulfate (Gentamicin Sulfate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Gentamicin Sulfate (Gentamicin Sulfate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Gentamicin Sulfate (Gentamicin Sulfate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Gentamicin Sulfate (Gentamicin Sulfate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Gentamicin Sulfate (Gentamicin Sulfate) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 03/2007