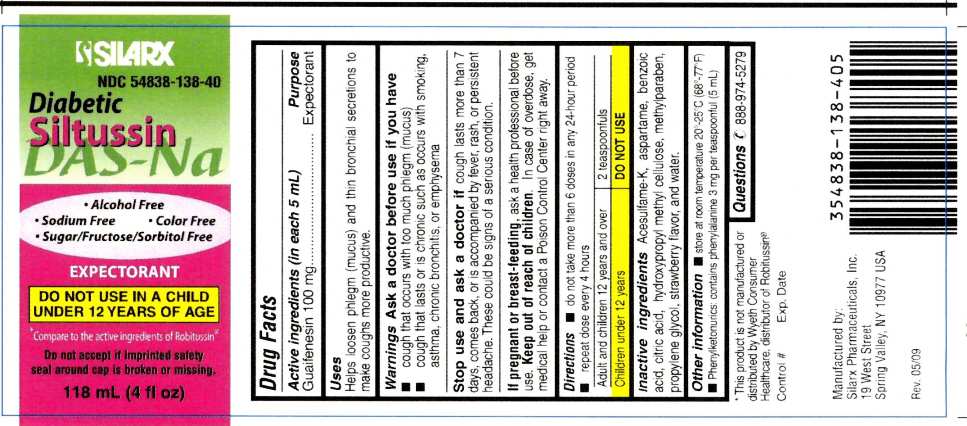
DIABETIC SILTUSSIN DAS-NA
-
guaifenesin liquid
Silarx Pharmaceuticals, Inc
----------
Diabetic Siltussin DAS-NaActive Ingredient: Guaifenesin 100 mg (in each 5 mL)
Purpose: Expectorant
Uses
Helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive.
Warnings
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Stop use and ask a doctor if cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- do not take more than 6 doses in any 24-hour period repeat dose every 4 hours
| Adult and children 12 years and over | 2 teaspoonfuls |
| Children under 12 years | DO NOT USE |
store at room temperature 20°-25°C (68°-77°F)
Phenylketonurics: contains phenylalanine 3 mg per teaspoonful (5 mL)
Inactive Ingredients
Acesulfame-K, aspartame, benzoic acid, citric acid, hydroxypropyl methyl cellulose, methylparaben, propylene glycol, strawberry flavor, and water.
Questions
888-974-5279
* This product is not manufactured or distributed by Wyeth Consumer Healthcare, distributor of Robitussin®
Manufactured by:
Silarx Pharmaceuticals, Inc.
19 West Street,
Spring Valley, NY 10977
USA
| DIABETIC SILTUSSIN DAS-NA
guaifenesin liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 12/05/2005 | |
| Labeler - Silarx Pharmaceuticals, Inc (161630033) |