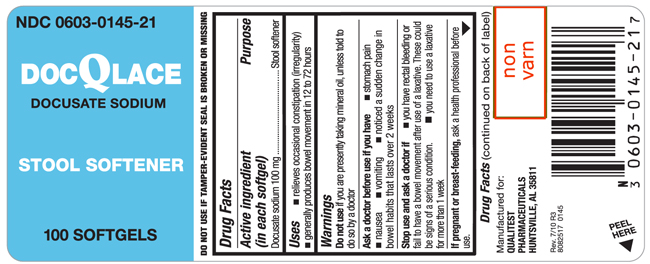
DOCQLACE
-
docusate sodium capsule
Qualitest Pharmaceuticals
----------
DocQLaceActive ingredient (in each softgel)
Docusate sodium 100 mg
Purpose
Stool softener
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 12 to 72 hours
Warnings
Do not use if you are presently taking mineral oil, unless told to do so by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Stop use and ask a doctor if
- you have rectal bleeding or fail to have a bowel movement after use of a laxative. These could be signs of a serious condition.
- you need to use a laxative for more than 1 week
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
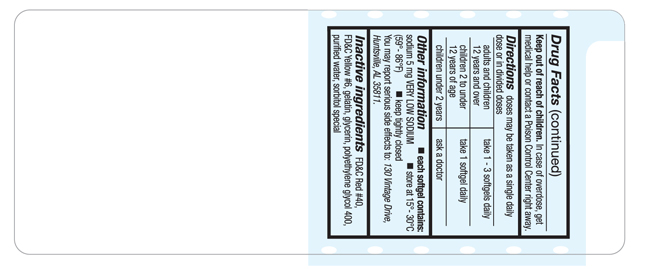
Directions
doses may be taken as a single daily dose or in divided doses
|
adults and children 12 years and over |
take 1-3 softgels daily |
|
children 2 to under 12 years of age |
take 1 softgel daily |
|
children under 2 years |
ask a doctor |
Other information
- each softgel contains: sodium 5 mg VERY LOW SODIUM
- store at 15°- 30°C (59°- 86°F)
- keep tightly closed
You may report serious side effects to: 130 Vintage Drive, Huntsville, AL 35811.
Inactive ingredients
FD&C Red #40, FD&C Yellow #6, gelatin, glycerin, polyethylene glycol 400, purified water, sorbitol special
Manufactured for:
QUALITEST PHARMACEUTICALS
HUNTSVILLE, AL 35811
Rev. 7/10 R3
8082517 0145
PRINCIPAL DISPLAY PANEL
| DOCQLACE
docusate sodium capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH NOT FINAL | part334 | 02/01/1996 | |
| Labeler - Qualitest Pharmaceuticals (011103059) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Swiss Caps USA, INC | 033105888 | ANALYSIS, MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Vintage Pharmaceuticals-Charlotte | 151228897 | LABEL, PACK | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Vintage Pharmaceuticals-Huntsville | 825839835 | LABEL, PACK | |