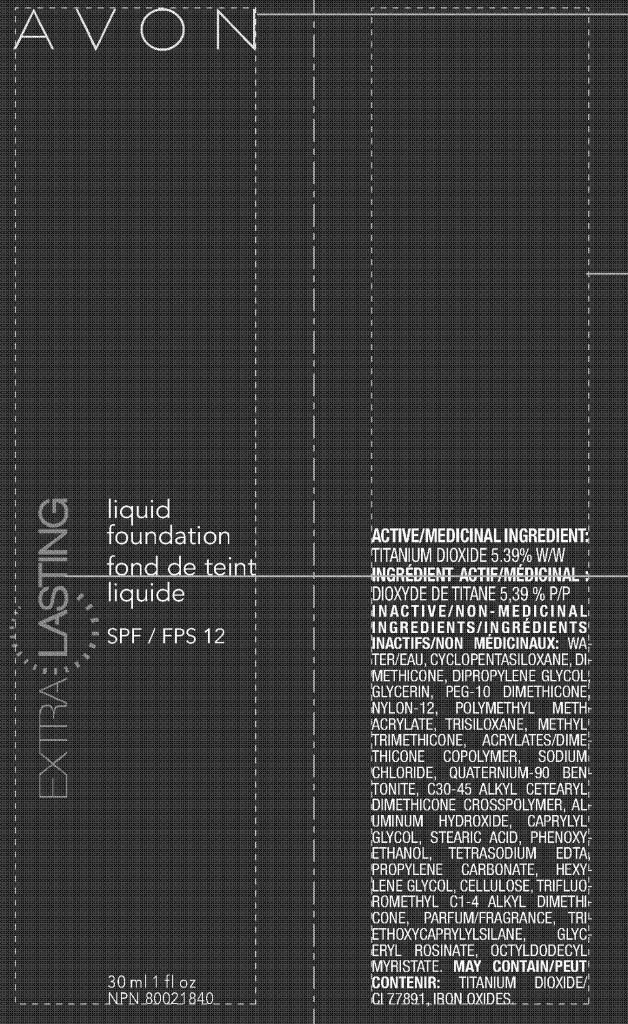
EXTRALASTING LIQUID FOUNDATION
-
titanium dioxide lotion
Avon Products, Inc.
----------
USES: Helps prevent
sunburn.
DIRECTIONS: Apply
evenly before sun exposure
and as needed. Children
under 6 months of age: Ask
a health care practitioner.
WARNINGS: For external
use only. When using this
product, keep out of eyes. If
contact occurs, rinse thoroughly
with water to remove. Stop
use and ask a health care
practitioner if rash or irritation
develops or lasts. Keep out
of reach of children. If swallowed,
get medical help or contact
a Poison Control Center
right away.
ACTIVE/MEDICINAL INGREDIENT:
TITANIUM DIOXIDE 5.39%
INACTIVE/NON-MEDICINAL
INGREDIENTS/INGRÉDIENTS
INACTIFS/NON MÉDICINAUX: WATER/
EAU, CYCLOPENTASILOXANE, DIMETHICONE,
DIPROPYLENE GLYCOL,
GLYCERIN, PEG-10 DIMETHICONE,
NYLON-12, POLYMETHYL METHACRYLATE,
TRISILOXANE, METHYL
TRIMETHICONE, ACRYLATES/DIMETHICONE
COPOLYMER, SODIUM
CHLORIDE, QUATERNIUM-90 BENTONITE,
C30-45 ALKYL CETEARYL
DIMETHICONE CROSSPOLYMER, ALUMINUM
HYDROXIDE, CAPRYLYL
GLYCOL, STEARIC ACID, PHENOXYETHANOL,
TETRASODIUM EDTA,
PROPYLENE CARBONATE, HEXYLENE
GLYCOL, CELLULOSE, TRIFLUOROMETHYL
C1-4 ALKYL DIMETHICONE,
PARFUM/FRAGRANCE, TRIETHOXYCAPRYLYLSILANE,
GLYCERYL
ROSINATE, OCTYLDODECYL
MYRISTATE. MAY CONTAIN/PEUT
CONTENIR: TITANIUM DIOXIDE/
CI 77891, IRON OXIDES.

| EXTRALASTING
LIQUID FOUNDATION
titanium dioxide lotion |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part352 | 12/15/2010 | |
| Labeler - Avon Products, Inc. (001468693) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Avon Products, Inc. | 005149471 | manufacture | |