ISOMETHEPTENE MUCATE, CAFFEINE, ACETAMINOPHEN
-
isometheptene mucate,
caffeine and
acetaminophen capsule
River's Edge Pharmaceuticals, LLC
----------
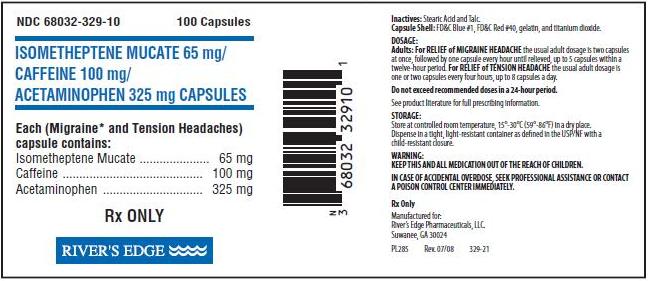
ISOMETHEPTENE MUCATE 65 mg/CAFFEINE 100 mg/ACETAMINOPHEN 325 mg CAPSULESDESCRIPTION:
Each red capsule contains Isometheptene Mucate 65 mg, Caffeine 100 mg, and Acetaminophen 325 mg.
Inactives: Stearic Acid and Talc.
Capsule Shell: FD&C Blue #1, FD&C Red #40, gelatin, and titanium dioxide.
Isometheptene Mucate is a white crystalline powder having a characteristic aromatic and bitter taste. It is an unsaturated aliphatic amine with sympathomimetic properties. Having the chemical name: Isometheptene, galactarate (2:1) (salt), 1, 5 Trimethyl-4-hexanylamine mucate.
Caffeine is a white crystalline powder with a bitter taste. Having the chemical name 1H-Purine-2,6-dione,3,7-dihydro-1,3,7-trimethyl-.
Acetaminophen, a non-salicylate, occurs as a white, odorless, crystalline powder, possessing a slightly bitter taste. Having the chemical name: Acetamide, N-(4-hydroxyphenyl)-.
ACTIONS:
Isometheptene Mucate, a sympathomimetic amine, and Caffeine act by constricting dilated cranial and cerebral arterioles, thus reducing the stimuli that lead to vascular headache. Acetaminophen raises the threshold to painful stimuli, thus exerting an analgesic effect against all types of headaches.INDICATIONS:
For the relief of tension and vascular headaches*.* Based on a review of this drug (isometheptene mucate) by the National Academy of Sciences – National Research Council and/or other information, FDA has classifed the other indication as “possibly” effective in the treatment of migraine headache. Final classification of the less-than-effective indication requires further investigation.
CONTRAINDICATIONS:
Isometheptene Mucate 65 mg/ Caffeine 100 mg/ Acetaminophen 325 mg Capsules are contraindicated in glaucoma and/or severe cases of renal disease, hypertension, organic heart disease, hepatic disease and in those patients who are on monoamine-oxidase (MAO) inhibitor therapy.PRECAUTIONS:
Caution should be observed in hypertension, peripheral vascular disease and after recent cardio-vascular attacks.ADVERSE REACTIONS:
Hypersensitive patients have shown rash and transient dizziness; this can be eliminated by reducing dosage.DOSAGE AND ADMINISTRATION
Adults: For RELIEF of MIGRAINE HEADACHE the usual adult dosage is two capsules at once, followed by one capsule every hour until relieved, up to 5 capsules within a twelve-hour period. For RELIEF of TENSION HEADACHE the usual adult dosage is one or two capsules every four hours, up to 8 capsules a day.Do not exceed recommended doses in a 24-hour period.
HOW SUPPLIED:
Red capsules imprinted with RE 329.Bottles of 100 capsules, NDC # 68032-329-10.
Store at a controlled room temperature 15°– 30° C (59° – 86° F) in a dry place.
Dispense in a tight, light-resistant container as defined in the USP/NF with a child-resistant closure.
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN.
IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Rx Only
Manufactured for:
River’s Edge Pharmaceuticals, LLC
Suwanee, GA 30024
PI 285 Rev. 07/08 329-11
PACKAGING
| ISOMETHEPTENE MUCATE, CAFFEINE, ACETAMINOPHEN
isometheptene mucate, caffeine, acetaminophen capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 07/01/2008 | 09/30/2010 | |
| Labeler - River's Edge Pharmaceuticals, LLC (133879135) |