COLDEC DS
-
carbinoxamine maleate and
pseudoephedrine hydrochloride syrup
Breckenridge Pharmaceutical, Inc.
----------
Coldec DS SyrupDESCRIPTION
Antihistamine/Decongestant for oral use
Each teaspoonful (5 mL) contains:
| Carbinoxamine Maleate | 2 mg |
| Pseudoephedrine Hydrochloride | 25 mg |
INACTIVE INGREDIENTS
Sodium Benzoate, Citric Acid, Glycerin, Sorbitol, Flavor and Purified Water.
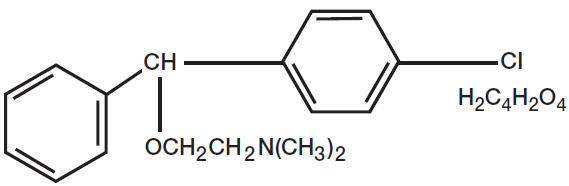
Carbinoxamine maleate (2-[p-chloro-a-[2-(dimethylamino)ethoxy]benzylpyridine maleate) is one of the ethanolamine class of H1 antihistamines.
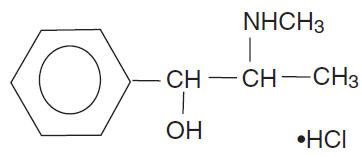
Pseudoephedrine hydrochloride (Benzenemethanol, α-[1-(methylamino)ethyl]-,[S-(R*,R*)]-, hydrochloride) is the hydrochloride of pseudoephedrine, a naturally occurring dextrorotary stereoisomer of ephedrine.
CLINICAL PHARMACOLOGY
Antihistaminic and decongestant actions. Carbinoxamine maleate possesses H1 antihistaminic activity and mild anticholinergic and sedative effects. Serum half-life for carbinoxamine is estimated to be 10-20 hours. Virtually no intact drug is excreted in the urine.
Pseudoephedrine hydrochloride is an oral sympathomimetic amine that acts as a decongestant to respiratory tract mucous membranes. While its vasoconstrictor action is similar to that of ephedrine, pseudoephedrine has less pressor effect in normotensive adults. Serum half-life for pseudoephedrine is 6 to 8 hours. Acidic urine is associated with faster elimination of the drug. About one half of the administered dose is excreted in the urine.
INDICATIONS AND USAGE
For symptomatic relief of seasonal and perennial allergic rhinitis and vasomotor rhinitis. Coldec DS Syrup is an immediate-release dosage form allowing titration of dose up to four times a day.
CONTRAINDICATIONS
Patients with hypersensitivity or idiosyncrasy to ingredients, patients taking monoamine oxidase (MAO) inhibitors, patients with narrow-angle glaucoma, urinary infection, peptic ulcer, severe hypertension or coronary artery disease, or patients undergoing an asthmatic attack.
WARNINGS
Use in Pregnancy
Pregnancy category C
Safety for use during pregnancy has not been established.
Nursing Mothers
Use with caution in nursing mothers.
Special Risk Patients
Use with caution in patients with hypertension or ischemic heart disease, and persons older than 60 years.
PRECAUTIONS
Use with caution in patients with hypertension, heart disease, asthma, hyperthyroidism, increased intraocular pressure, diabetes melitus and prostatic hypertrophy.
Information for patients
Avoid alcohol and other CNS depressants while taking these products. Patients sensitive to antihistamines may experience moderate to severe drowsiness. Patients sensitive to sympathomimetic amines may note mild CNS stimulation. When taking these products, exercise care in driving or operating appliances, machinery, etc.
Drug interactions
antihistamines may enhance the effects of tricyclic antidepressants, barbiturates, alcohol, and other CNS depressants. MAO inhibitors prolong and intensify the anticholinergic effects of antihistamines. Sympathomimetic amines may reduce the antihypertensive effects of reserpine, veratrum alkaloids, methyldopa and mecamylamine. Effects of sympathomimetics are increased with MAO inhibitors and beta-adrenergic blockers.
Pregnancy Category C
Animal Reproduction studies have not been conducted with these products. It is also not known whether these products can cause fetal harm when administered to a pregnant woman or effect reproduction capacity. Give to a pregnant woman only if clearly needed.
ADVERSE REACTIONS
Antihistamines: sedation, dizziness, diplopia, vomiting, diarrhea, dry mouth, headache, nervousness, nausea, anorexia, heartburn weakness, polyuria and dysuria and, rarely, excitability in children.
Sympathomimetic amines: Convulsions, CNS stimulation, cardiac arrhythmias, respiratory difficulty, increased heart rate or blood pressure, hallucinations, tremors, nervousness, insomnia, weakness, pallor and dysuria.
OVERDOSAGE
No information is available as to specific results of an overdose on these products. The signs, symptoms, and treatment described below are those of H1 antihistamines and ephedrine overdose.
Symptoms
Should antihistamine effects predominate, central action constitutes the greatest danger. In a small child, symptoms include excitation, hallucination, ataxia, incoordination, tremors, flushed face and fever. Convulsions, fixed and dilated pupils, coma and death may occur in severe cases. In the adult, fever and flushing are uncommon; excitement leading to convulsions and postictal depression is often preceded by drowsiness and coma. Respiration is usually not seriously depressed; blood pressure is usually stable.
Should sympathomimetic symptoms predominate, central effects include restlessness, dizziness, tremor, hyperactive reflexes, talkativeness, irritability and insomnia. Cardiovascular and renal effects include difficulty in micturition, headache, flushing, palpitation, cardiac arrhythmias, hypertension with subsequent hypotension and circulatory collapse. Gastrointestinal effects include dry mouth, metallic taste, anorexia, nausea, vomiting, diarrhea and abdominal cramps.
Treatment
a) Evacuate stomach as condition warrants. Activated charcoal may be useful. b) maintain a non-stimulating environment. c) monitor cardiovascular status. d) Do not give stimulants. e) Reduce fever with cool sponging. f) Support respiration. g) Use sedatives or anticonvulsants to control CNS excitation and convulsions. h) Physostigmine may reverse anticholinergic symptoms. i) ammonium chloride may acidify the urine to increase excretion of pseudoephedrine. j) further care is symptomatic and supportive.
DOSAGE AND ADMINISTRATION
| AGE | DOSAGE | FREQUENCY* |
|---|---|---|
|
||
| Adults and Children 6 years and over | 2 teaspoons | q.i.d. |
| Children 18 months to 6 years | 1 teaspoon | q.i.d. |
| Under 18 months of age | ||
| 9-18 months | 3/4 - 1 teaspoon | |
| 6-9 months | 3/4 - teaspoon | |
| 3-6 months | 1/2 - teaspoon | |
| 1-3 months | 1/4 - teaspoon | |
| (titrate dosage individually) | ||
HOW SUPPLIED
Coldec DS is a strawberry/pineapple fruit flavored syrup available in 16 fl. oz. (1 pint) bottles.
NDC 51991-309-16
Dispense in USP tight container.
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). See USP controlled room temperature.
Rx Only
Manufactured for:
Breckenridge Pharmaceutical, Inc.
Boca Raton, FL 33487
Manufactured by:
Tri-Med Laboratories, Inc.
Somerset NJ 08873
REV. 3/03
PRINCIPAL DISPLAY PANEL - 473 ml Bottle Label
Breckenridge
Pharmaceutical, Inc.
NDC 51991-309-16
New
Formula
Coldec DS
Antihistamine/Decongestant
• Dye Free • Sugar Free
• Alcohol Free
Description: Each teaspoonful (5 mL)
contains:
Carbinoxamine Maleate 2 mg
Pseudoephedrine HCl 25 mg
INACTIVE INGREDIENTS: Sodium
Benzoate, Citric Acid, Glycerin,
Sorbitol, Flavor and Purified Water.
Rx Only
Net Contents:
16 fl. oz. (One Pint) 473 ml

| COLDEC DS
carbinoxamine maleate and pseudoephedrine hydrochloride syrup |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED DRUG OTHER | 05/01/2003 | 03/31/2004 | |
| Labeler - Breckenridge Pharmaceutical, Inc. (150554335) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Trimed | 182050567 | MANUFACTURE | |