CARBETAPLEX
-
guaifenesin,
carbetapentane citrate and
phenylephrine hydrochloride liquid
Breckenridge Pharmaceutical, Inc.
----------
Carbetaplex™ LiquidAntitussive, Decongestant & Expectorant
DESCRIPTION
Carbetaplex™ Liquid is an sugar free, dye free, alcohol free liquid with a strawberry odor and flavor for oral administration.
Each teaspoonful (5 mL) contains:
| Guaifenesin | 100 mg |
| Carbetapentane Citrate | 20 mg |
| Phenylephrine Hydrochloride | 15 mg |
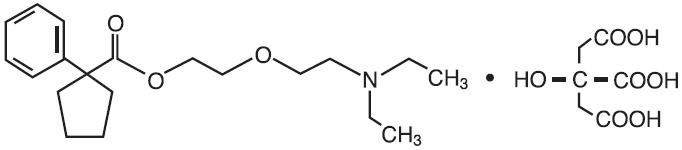
Carbetapentane Citrate is a centrally acting non-narcotic antitussive. Chemically it is 1- Phenylcyclopentanecarboxylic acid 2-(2-diethylaminoethoxy)ethyl ester citrate. The molecular weight is 525.59 and the molecular formula is C20H31NO3 • C6H8O7. Carbetapentane Citrate is a white crystalline powder. It is freely soluble in water and chloroform. Its structure is as follows:
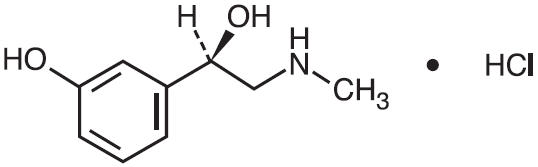
Phenylephrine Hydrochloride is a decongestant. The chemical name is (-)-m-Hydroxy-α -[(methylamino)methyl]benzyl alcohol hydrochloride. The molecular weight is 203.67 and the molecular formula is C9H13NO2 • HCl. Phenylephrine hydrochloride occurs as white or practically white, odorless crystals, having a bitter taste. It is freely soluble in water and in alcohol. Its structure is as follows:
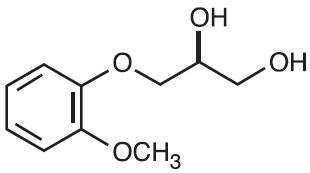
Guaifenesin is an expectorant. The chemical name is 1,2-Propanediol, 3-(2-methoxyphenoxy)-,(±)-. The molecular weight is 198.22 and the molecular formula is C10H14O4.Guaifenesin occurs as a white to slightly gray crystalline powder, having a bitter taste. It is freely soluble in alcohol; soluble in water, chloroform, glycerin, and propylene glycol. Its structure is as follows:
Carbetaplex™ Liquid also contains Sodium Benzoate, Citric Acid, Saccharin Sodium, Propylene Glycol, Sorbitol Solution, Natural Strawberry Flavor and Purified Water.
CLINICAL PHARMACOLOGY
Carbetapentane Citrate
Carbetapentane is a centrally acting non-narcotic antitussive.
Phenylephrine Hydrochloride
Phenylephrine is a sympathomimetic which acts predominantly on alpha receptors and has little action on beta receptors. It, therefore, functions as an oral nasal decongestant with minimal CNS stimulation.
Guaifenesin
Guaifenesin has an expectorant action which increases the output of respiratory tract fluid by reducing adhesiveness and surface tension. Sinus and bronchial drainage is improved and dry, non-productive coughs become more productive and less frequent.
INDICATIONS
Carbetaplex™ Liquid is indicated for temporary relief of non-productive cough accompanying respiratory tract congestion and discomfort associated with the common cold, influenza and bronchitis.
CONTRAINDICATIONS
Carbetaplex™ Liquid is contraindicated in newborns, infants, nursing mothers and in patients with a known hypersensitivity to any of its ingredients. It is also contraindicated in patients with severe hypertension, severe coronary artery disease, hyperthyroidism, and in patients on MAO inhibitor therapy (or for 14 days after stopping MAOI therapy). Patient idiosyncrasy to adrenergic agents may be manifested by insomnia, dizziness, weakness, tremor, or arrhythmias.
WARNINGS
Do not exceed recommended dosage. If nervousness, dizziness, or sleeplessness occur, discontinue use and consult a doctor. If symptoms do not improve within 7 days or are accompanied by a fever, consult a doctor.
General
Sympathomimetic amines should be used with caution in patients with hypertension, diabetes mellitus, ischemic heart disease, increased intraocular pressure, hyperthyroidism, or prostatic hypertrophy. Sympathomimetics may produce central nervous system stimulation with convulsions or cardiovascular collapse with accompanying hypotension.
Hypertensive crisis can occur with concurrent use of phenylephrine and monoamine oxidase (MAO) inhibitors (or for 14 days after stopping MAOI therapy), indomethacin or with beta- blockers and methyldopa. If a hypertensive crisis occurs, these drugs should be discontinued immediately and therapy to lower blood pressure should be instituted. Fever should be managed by means of external cooling.
PRECAUTIONS
Drug Interactions
The use of MAO inhibitors (or for 14 days after stopping MAOI therapy) and β-adrenergic blockers may prolong and intensify the over all effects of sympathomimetic agents. Sympathomimetics may reduce the hypotensive effects of guanethidine, mecamylamine, methyldopa, reserpine, and veratrum alkaloids. Concurrent use of tricyclic antidepressants may antagonize the effects of phenylephrine. Use of other vasopressor drugs during halothane anesthesia may cause serious cardiac arrhythmias. Guaifenesin may produce an increase in urinary 5-hydroxyindoleacetic acid and may therefore interfere with the interpretation of this test for the diagnosis of carcinoid syndrome. It may also falsely elevate the VMA test for catechols. Administration of this drug should be discontinued 48 hours prior to the collection of urine specimens for such tests.
Stimulants, such as phenylephrine, are banned and tested for by the U.S. Olympic Committee (USOC) and the National Collegiate Athletic Association (NCAA).
Carcinogenesis, Mutagenesis, Impairment of Fertility
No adequate and well-controlled studies have been conducted to determine whether the components of Carbetaplex™ Liquid have a potential for carcinogenesis, mutagenesis, or impairment of fertility.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with Carbetaplex™ Liquid. It is also not known whether it can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. This product should not be administered to pregnant women.
Nursing Mothers
Due to the possible passage of the ingredients into breast milk, this product should not be given to nursing mothers.
Pediatric Use
Safety and effectiveness in the pediatric population, under 6, have not been established.
Geriatric Use
The elderly (60 years and older) are more likely to have adverse reactions to sympathomimetics. Overdose of sympathomimetics in this age group may cause hallucinations, convulsions, CNS depression and death.
ADVERSE REACTIONS
Sympathomimetic drugs have been associated with certain untoward reactions including fear, anxiety, tenseness, restlessness, tremor, weakness, pallor, respiratory difficulty, dysuria, insomnia, hallucinations, convulsions, CNS depression, arrhythmias, and cardiovascular collapse with hypotension. Mild central nervous system stimulation, especially in those patients who are hypersensitive to sympathomimetic drugs, may occur. Nervousness, excitability, restlessness, dizziness, weakness, and insomnia may also occur. Headache and drowsiness have also been reported. Large doses may cause light-headedness, nausea and/or vomiting. Patient idiosyncrasy to adrenergic agents may be manifested by insomnia, dizziness, weakness, tremor or arrhythmias.
Guaifenesin is well tolerated and has a wide margin of safety. Side effects have been generally mild and infrequent. Nausea and vomiting are the side effects that occur most commonly. Dizziness, headache, and rash (including urticaria) have been reported rarely.
OVERDOSAGE
Signs and Symptoms
Central effects include restlessness, dizziness, tremor, hyperreactive reflexes, talkativeness, irritability, and insomnia. Cardiovascular and renal effects include difficulty in urination, headache, flushing, palpitation, cardiac arrhythmias, hypertension with subsequent hypotension and circulatory collapse. Gastrointestinal effects include dry mouth, anorexia, nausea, vomiting, diarrhea, and abdominal cramps.
Treatment
The patient should be induced to vomit, even if emesis has occurred spontaneously. Pharmacologic vomiting by the administration of ipecac syrup is a preferred method, however, vomiting should not be induced in patients with impaired consciousness or loss of gag reflex. Precautions against aspiration must be taken, especially in infants, children and comatose patients. Following emesis, any drug remaining in the stomach may be absorbed by activated charcoal administered as a slurry with water. Treatment of the signs and symptoms of overdosage is symptomatic and supportive.
DOSAGE AND ADMINISTRATION
Adults and children over 12 years of age: 1 teaspoonful (5 mL) every 4-6 hours. Children 6 to 12 years: 1/2 teaspoonful (2.5 mL) every 4-6 hours. This product is not indicated for use in children under 6 years of age. (see PRECAUTIONS, Pediatric Use.) Do not exceed four doses in a 24 hour period.
HOW SUPPLIED
Carbetaplex™ Liquid is supplied as a sugar free, dye free, alcohol free, clear, strawberry flavored liquid, in bottles of 16 fl.oz. (One Pint), NDC 51991-083-16.
WARNING: KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Storage
Store at 25°C(77°F); excursions permitted to 15°-30°C(59°-86°F). See USP Controlled Room Temperature. Protect from freezing.
Pharmacist: Dispense in a tight, light-resistant container with a child-resistant closure as defined in the USP/NF.
All prescription substitutions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product.
Rx ONLY
Distributed by:
Breckenridge Pharmaceutical, Inc.
Boca Raton, FL 33487
Manufactured by:
Tri-Med Laboratories, Inc.
Somerset, NJ 08873
Rev. 5/08
PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
Breckenridge
Pharmaceutical, Inc.
NDC 51991-083-16
Carbetaplex™
Liquid
Antitussive/Decongestant/
Expectorant
- Sugar Free
- Dye Free
- Alcohol Free
Description: Each 5 mL (one teaspoonful) for oral
administration contains:
| Guaifenesin | 100 mg |
| Carbetapentane Citrate | 20 mg |
| Phenylephrine Hydrochloride | 15 mg |
INACTIVE INGREDIENTS: Sodium Benzoate,
Citric Acid, Saccharin Sodium, Propylene Glycol,
Sorbitol Solution, Natural Strawberry Flavor,
Purified Water.
Rx Only
Net Contents:
16 fl. oz. (One Pint) 473 mL

| CARBETAPLEX
guaifenesin, carbetapentane citrate, and phenylephrine hydrochloride liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED DRUG OTHER | 04/01/2004 | 09/30/2009 | |
| Labeler - Breckenridge Pharmaceutical, Inc. (150554335) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Trimed | 182050567 | MANUFACTURE | |