CPM-PSE DM
-
chlorpheniramine maleate,
dextromethorphan hydrobromide and
pseudoephedrine hydrochloride liquid
TRIGEN Laboratories, Inc.
----------
CPM/PSE DM DropsDrug Facts
Active Ingredients
Each 1 mL dropperful of grape flavored liquid for oral administration contains:
| 0.8 mg | Chlorpheniramine Maleate | |
| 3 mg | Dextromethorphan HBr | |
| 9 mg | Pseudoephedrine HCl |
Purpose
- Antihistamine
- Cough Suppressant
- Decongestant
Chlorpheniramine Maleate is an antihistamine having the chemical name: 2-Pyridinepropanamine, γ-(4-chlorphenyl)- N,N-dimethyl-(Z)-2-butenedioate (1:1). , and has the following chemical formula:
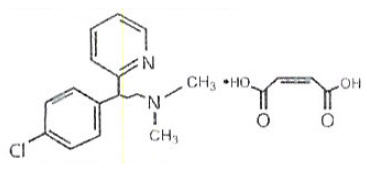
C16H19ClN2 • C4H4O4 M.W. 390.86
Dextromethorphan Hydrobromide is a salt of the methyl ether of the dextrorotatory isomer of levorphanol, a narcotic analgesic. Chemically, it is Morphinan, 3-methoxy-17- methyl-, (9α, 13α, 14α)-, hydrobromide, monohydrate. Its structure is as follows:
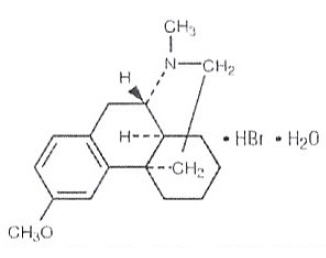
C18H25NO • HBr • H2O M.W. 370.32
Pseudoephedrine hydrochloride is an adrenergic (vasoconstrictor) which occurs as fine, white to off white crystals or powder, having a faint characteristic odor. It is very soluble in water, freely soluble in alcohol and sparing soluble in chloroform. The chemical name is: benzenemethanol, α-[1-(methylamino)ethyl]-[ S-(R*,R*)-, hydrochloride. Its structure is as follows:
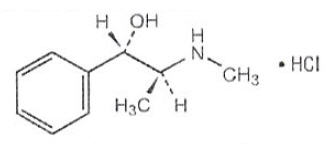
C10H15NO • HCl M.W. 201.69
CLINICAL PHARMACOLOGY
Chlorpheniramine
Chlorpheniramine Maleate is an alkylamine-type antihistamine that possesses anticholineric and sedative effects. Antihistamines competitively antagonize histamine at the H1 receptor site. Thus, activation of H1 receptors by released histamine which results in increased vascular permeability, increased mucus production, pruritus and sneezing is prevented.
Dextromethorphan
Dextromethorphan Hydrobromide is an antitussive agent which, unlike the isomeric levorphanol, has no analgesic or addictive properties. The drug acts centrally and elevates the threshold for coughing. It is about equal to codeine in depressing the cough reflex. In therapeutic dosage, Dextromethorphan Hydrobromide does not inhibit ciliary activity. Dextromethorphan Hydrobromide is rapidly absorbed from the gastrointestinal tract, metabolized by the liver and excreted primarily in the urine.
Pseudoephedrine
Pseudoephedrine acts as an indirect sympathomimetic agent by stimulating sympathetic (adrenergic) nerve endings to release norepinephrine. Norepinephrine in turn stimulates alpha and beta receptors throughout the body. The action of Pseudoephedrine is apparently more specific for the blood vessels of the upper respiratory tract and less specific for the blood vessels of the systemic circulation. The vasoconstriction elicited at these sites results in the shrinkage of swollen tissues in the sinuses and nasal passages. Pseudoephedrine is rapidly and almost completely absorbed from the gastrointestinal tract. Considerable variation in half-life has been observed (from about 4 1/2 to 10 hours), which is attributed to individual differences in absorption and excretion. Excretion rates are also altered by urine pH, increasing with acidification and decreasing with alkalinization. As a result, mean half-life falls to about 4 hours at pH 5 and increases to 12 to 13 hours at pH 8.
INDICATIONS
Temporarily relieves symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies; runny nose, sneezing, itching of the nose or throat, itchy, watery eyes, cough due to minor throat and bronchial irritation, nasal congestion, reduces swelling of nasal passages.
WARNINGS
Do not exceed recommended dosage.
Do not use this product under 4 years of age.
Do not use this product in a child who is taking a prescription monoamine oxidase inhibitor (MAOI)(certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if the child has a breathing problem such as chronic bronchitis, glaucoma, a persistent or chronic cough such that occurs with asthma, a cough that occurs with too much phlegm (mucus), heart disease, high blood pressure, thyroid disease, diabetes mellitus.
Ask a doctor or pharmacist before use if the child is taking sedatives or tranquilizers.
When using this product excitability may occur, especially in children; may cause marked drowsiness; sedatives and tranquilizers may increase drowsiness.
Stop use and ask a doctor if nervousness, dizziness, or sleeplessness occur; cough or nasal congestion persists for more than 1 week, tends to recur, or is accompanied by a fever, rash or persistent headache. These could be signs of a serious condition. New symptoms occur.
Keep out of the reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away.
DOSAGE1 AND ADMINISTRATION
Administer each dose four times per day using provided dropper. Do not exceed recommended dosage.
- 1
- In mild cases or in particularly sensitive patients, less frequent or reduced doses may be adequate.
Children 6 to under 12 years
2 dropperfuls (2 mL) every 4-6 hours, not to exceed 4 doses (8 dropperfuls) per 24 hours.
Children under 6 years of age
consult a physician.
Also contains the following inactive ingredients (in alphabetical order): Citric Acid, FD&C Blue #1, FD&C Red #40, Glycerin, Grape flavor, Methyl Paraben, Potassium Citrate, Potassium Sorbate, Propyl Paraben, Propylene Glycol, Purified Water, Sorbitol Solution 70%, Sucralose
Store at controlled room temperature 15°-30°C (59°-86°F).
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Supplied in a tight, light-resistant container with a child-resistant cap.
Manufactured for:
TRIGEN
LABORATORIES
Sayreville, NJ 08872
Rev. 03/10
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Label
NDC 13811-072-30
CPM/PSE DM
Drops
- Antihistamine
- Cough Suppressant
- Decongestant
Each 1 mL dropperful of
grape-flavored liquid for oral
administration contains:
| Chlorpheniramine Maleate | 0.8 mg |
| Dextromethorphan HBr | 3 mg |
| Pseudoephedrine HCl | 9 mg |
1 fl oz (30 mL)

| CPM-PSE DM
chlorpheniramine maleate, dextromethorphan hydrobromide, and pseudoephedrine hydrochloride liquid |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| Unapproved drug other | 03/01/2010 | ||
| Labeler - TRIGEN Laboratories, Inc. (830479668) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| TG United Liquids, Inc. | 963714766 | MANUFACTURE | |