DECONSAL CT
-
phenylephrine hydrochloride and
pyrilamine maleate tablet, chewable
Cornerstone Therapeutics Inc.
----------
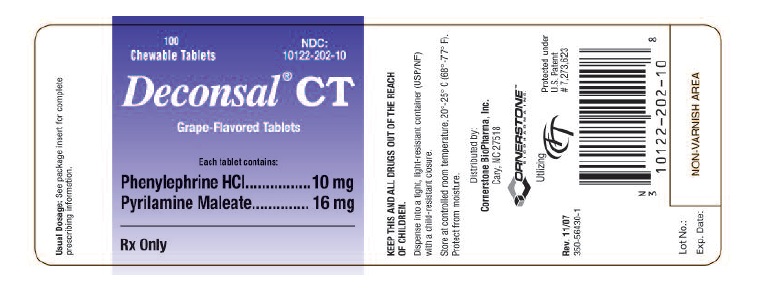
Deconsal® CTChewable Tablets
DESCRIPTION
Deconsal® CT Chewable Tablets are an antihistamine/nasal decongestant combination for oral administration as a chewable tablet.
Each Deconsal® CT Chewable Tablet in a grape-flavored, dye-free, mottled brown-colored, scored tablet for oral administration contains:
Phenylephrine HCl…….….……10 mg
Pyrilamine Maleate…….………16 mg
Inactive ingredients: Calcium phosphate dibasic, compressible sugar, corn starch, grape flavor, hypromellose, magnesium aluminum silicate, magnesium stearate, microcrystalline cellulose, saccharin sodium, talc, tannic acid, and xanthan gum.
See Dosage and Administration section for further descriptive composition.
CLINICAL PHARMACOLOGY
Deconsal® CT Chewable Tablets combines the sympathomimetic decongestant effect of phenylephrine with the antihistaminic action of pyrilamine.
PHENYLEPHRINE
Phenylephrine is a decongestant which is a potent postsynaptic α-receptor agonist with little effect on β receptors of the heart. A direct action at the receptors accounts for the greater part of its effects, only a small part being due to its ability to release norepinephrine. Phenylephrine has no effect on β-adrenergic receptors of the bronchi or peripheral blood vessels.
Phenylephrine has a mild stimulant effect.
PYRILAMINE
Pyrilamine is an antihistamine, H1 receptor blocking agent belonging to the ethylenediamine class of antihistamines. H1-blocking drugs inhibit the actions of histamine on smooth muscle, capillary permeability, and can both stimulate and depress the central nervous system. Pyrilamine also possesses anticholinergic and sedative properties.
INDICATIONS AND USAGE
Deconsal® CT Chewable Tablets are indicated for the symptomatic relief of coryza, nasal congestion associated with the common cold, sinusitis, allergic rhinitis, and other upper respiratory tract conditions. Appropriate therapy should be provided for the primary disease.
CONTRAINDICATIONS
Deconsal® CT Chewable Tablets are contraindicated in patients sensitive to any of the ingredients or related compounds. Antihistamines are contraindicated for use in the treatment of lower respiratory tract symptoms, including asthma.
Phenylephrine is contraindicated in patients with hypertension or with peripheral vascular insufficiency (ischemia may result with risk of gangrene or thrombosis of compromised vascular beds).
Deconsal® CT Chewable Tablets should not be used in patients receiving a monoamine oxidase inhibitor (MAOI) (see “PRECAUTIONS-DRUG INTERACTIONS”).
WARNINGS
This product contains an antihistamine that may cause drowsiness and may have additive central nervous system (CNS) effects with alcohol or other CNS depressants (e.g., hypnotics, sedatives, tranquilizers). Antihistamines should be used with caution in patients with stenosing peptic ulcer, pyloroduodenal obstruction, and urinary bladder obstruction due to symptomatic prostatic hypertrophy and narrowing of the bladder neck.
PRECAUTIONS
GENERAL
Antihistamines are more likely to cause dizziness, sedation, and hypotension in elderly patients and therefore should be used with caution. Antihistamines may cause excitation, particularly in pediatric patients, but their combination with sympathomimetics may cause either mild stimulation or mild sedation.
Use with caution in patients with hypertension, cardiovascular disease, hyperthyroidism, diabetes, or narrow angle glaucoma.
INFORMATION FOR PATIENTS
Caution patients against drinking alcoholic beverages or engaging in potentially hazardous activities requiring alertness, such as driving a car or operating machinery, while using this product. Patients should be warned not to use this product if they are now taking a prescription monoamine oxidase (MAO) inhibitor (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAO inhibitor drug. If patients are uncertain whether a prescription drug contains an MAO inhibitor, they should be instructed to consult a health professional before taking this product.
DRUG INTERACTIONS
MAO inhibitors may prolong and intensify the anticholinergic effects of sympathomimetic agents. Patients may develop hyperpyrexia, hypotension, nausea, myoclonic leg jerks, and coma following coadministration of MAO inhibitors and dextromethorphan. Thus, concomitant administration of Deconsal® CT Chewable Tablets and MAO inhibitors should be avoided (see “CONTRAINDICATIONS”).
CARCINOGENISIS, MUTAGENESIS, IMPAIRMENT OF FERTILITY
No long-term animal studies have been performed wth Deconsal® CT Chewable Tablets.
PREGNANCY
Teratogenic Effects- Pregnancy Category C
Animal reproduction studies have not been conducted with Deconsal® CT Chewable Tablets. It is also not known whether Deconsal® CT Chewable Tablets can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Deconsal® CT Chewable Tablets should be given to a pregnant woman only if clearly needed.
LABOR AND DELIVERY
Administration of phenylephrine to patients in late pregnancy or labor may cause fetal anoxia or bradycardia by increasing contractility of the uterus and decreasing uterine blood flow.
NURSING MOTHERS
Because of the higher risk of intolerance of antihistamines in small infants generally, and in newborns and prematures in particular, Deconsal® CT Tablets should not be administered to a nursing mother.
ADVERSE REACTIONS
The most common effects associated with antihistamines have been drowsiness sedation, dryness of mucous membranes, and gastrointestinal effects. Serious side effects with oral antihistamines, sympathomimetics, and antitussives have been rare.
Other adverse reactions may include:
Dermatologic – urticaria, drug rash, photosensitivity, pruritus.
Cardiovascular – hypotension, hypertension, cardiac arrhythmias, palpitations.
Central Nervous Systems (CNS) – disturbed coordination, tremor, irritability, insomnia, visual disturbances, weakness, nervousness, convulsion, headache, euphoria, and dysphoria.
Genitourinary – urinary frequency, difficult urination.
Gastrointestinal – epigastric discomfort, anorexia, nausea, vomiting, diarrhea, constipation.
Respiratory – tightness of the chest and wheezing, shortness of breath.
Hematologic – hemolytic anemia, thrombocytopenia, agranulocytosis.
OVERDOSAGE
SIGNS AND SYMPTOMS
May vary from CNS depression to stimulation (restlessness to convulsions). Antihistamine overdosage in young children may lead to convulsions and death. Atropine-like signs and symptoms may be prominent.
TREATMENT
Induce vomiting if it has not occurred spontaneously. Precautions must be taken against aspiration especially in infants, children, and comatose patients. If gastric lavage is indicated, isotonic or half-isotonic saline solution is preferred. Stimulants should not be used. If hypotension is a problem, vasopressor agents may be considered.
DOSAGE AND ADMINISTRATION
Deconsal® CT Chewable Tablets: Administer up to 4 tablets per day for patients 12 years and older and up to 2 tablets per day for patients 6 to 12 years of age.
HOW SUPPLIED
Deconsal® CT Chewable Tablets are available as grape-flavored, dye-free, mottled brown-colored, scored tablet imprinted with CC on one side and the score line on the other
NDC No.: 10122-202-10 – Bottles of 100 Chewable Tablets and NDC No.: 10122-202-02 single tablet sample pouches.
Store at controlled room temperature, 20º-25º C (68º -77º F). Dispense in a tight, light-resistantcontainer (USP/NF) with a child-resistant closure.
Protect from moisture.
Rx Only
Manufactured for:
Cornerstone BioPharma, Inc.
Cary, NC 27518
This product is licensed and protected under U.S. Patent number 7,273,623 issued 9/25/2007.
550-56430-1
CBD305B1107
Deconsal CT
| DECONSAL CT
deconsal ct tablet, chewable |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED DRUG OTHER | 10/01/2006 | 07/01/2009 | |
| Labeler - Cornerstone Therapeutics Inc. (153886994) |
| Registrant - Cornerstone Therapeutics Inc. (153886994) |