lexiva (fosamprenavir calcium) tablet
[GlaxoSmithKline]
DESCRIPTION
LEXIVA (fosamprenavir calcium) is a prodrug of amprenavir, an inhibitor of human immunodeficiency virus (HIV) protease. The chemical name of fosamprenavir calcium is (3S)-tetrahydrofuran-3-yl (1S,2R)-3-[[(4-aminophenyl) sulfonyl](isobutyl)amino]-1-benzyl-2-(phosphonooxy) propylcarbamate monocalcium salt. Fosamprenavir calcium is a single stereoisomer with the (3S)(1S,2R) configuration. It has a molecular formula of C25H34CaN3O9PS and a molecular weight of 623.7. It has the following structural formula:
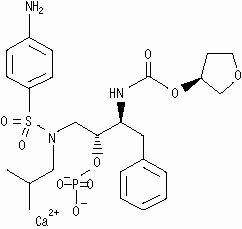
Fosamprenavir calcium is a white to cream-colored solid with a solubility of approximately 0.31 mg/mL in water at 25°C.
LEXIVA Tablets are available for oral administration in a strength of 700 mg of fosamprenavir as fosamprenavir calcium (equivalent to approximately 600 mg of amprenavir). Each 700-mg tablet contains the inactive ingredients colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and povidone K30. The tablet film-coating contains the inactive ingredients hypromellose, iron oxide red, titanium dioxide, and triacetin.
MICROBIOLOGY
Mechanism of Action
Fosamprenavir is rapidly converted to amprenavir by cellular phosphatases in vivo. Amprenavir is an inhibitor of HIV-1 protease. Amprenavir binds to the active site of HIV-1 protease and thereby prevents the processing of viral Gag and Gag-Pol polyprotein precursors, resulting in the formation of immature non-infectious viral particles.
Antiviral Activity
Fosamprenavir has little or no antiviral activity in vitro. The in vitro antiviral activity of amprenavir was evaluated against HIV-1 IIIB in both acutely and chronically infected lymphoblastic cell lines (MT-4, CEM-CCRF, H9) and in peripheral blood lymphocytes. The 50% effective concentration (EC50) of amprenavir ranged from 0.012 to 0.08 μM in acutely infected cells and was 0.41 μM in chronically infected cells (1 μM = 0.50 mcg/mL). The median EC50 value of amprenavir against HIV-1 isolates from clades A to G was 0.00095 µM in peripheral blood mononuclear cells (PBMCs). Similarly, the EC50 values for amprenavir against monocytes/macrophage tropic HIV-1 isolates (clade B) ranged from 0.003 to 0.075 µM in monocyte/macrophage cultures. The EC50 values of amprenavir against HIV-2 isolates grown in PBMCs were higher than those for HIV-1 isolates, and ranged from 0.003 to 0.11 µM. Amprenavir exhibited synergistic anti−HIV-1 activity incombination with the nucleoside reverse transcriptase inhibitors (NRTIs) abacavir, didanosine, lamivudine, stavudine, tenofovir, and zidovudine; the non-nucleoside reverse transcriptase inhibitors (NNRTIs) delavirdine and efavirenz; and the protease inhibitors (PIs) atazanavir and saquinavir. Amprenavir exhibited additive anti−HIV-1 activity in combination with the NNRTI nevirapine, the PIs indinavir, lopinavir, nelfinavir, and ritonavir; and the fusion inhibitor enfuvirtide. These drug combinations have not been adequately studied in humans.
Resistance
HIV-1 isolates with decreased susceptibility to amprenavir have been selected in vitro and obtained from patients treated with fosamprenavir. Genotypic analysis of isolates from treatment-naive patients failing amprenavir-containing regimens showed mutations in the HIV-1 protease gene resulting in amino acid substitutions primarily at positions V32I, M46I/L, I47V, I50V, I54L/M, and I84V, as well as mutations in the p7/p1 and p1/p6 Gag and Gag-Pol polyprotein precursor cleavage sites. Some of these amprenavir resistance-associated mutations have also been detected in HIV-1 isolates from antiretroviral-naive patients treated with LEXIVA. Of the 488 antiretroviral-naive patients treated with LEXIVA or LEXIVA/ritonavir in studies APV30001 and APV30002, respectively, 61 patients (29 receiving LEXIVA and 32 receiving LEXIVA/ritonavir) with virologic failure (plasma HIV-1 RNA >1,000 copies/mL on 2 occasions on or after Week 12) were genotyped. Five of the 29 antiretroviral-naive patients (17%) receiving LEXIVA without ritonavir in study APV30001 had evidence of genotypic resistance to amprenavir: I54L/M (n = 2), I54L + L33F (n = 1), V32I + I47V (n = 1), and M46I + I47V (n = 1). No amprenavir resistance-associated mutations were detected in antiretroviral-naive patients treated with LEXIVA/ritonavir for 48 weeks in study APV30002. However, the M46I and I50V mutations were detected in isolates from 1 virologic failure patient receiving LEXIVA/ritonavir once daily at Week 160 (HIV-1 RNA >500 copies/mL). Upon retrospective analysis of stored samples using an ultrasensitive assay, these resistant mutants were traced back to Week 84 (76 weeks prior to clinical virologic failure).
Cross-Resistance
Varying degrees ofcross-resistance among HIV-1 protease inhibitors have been observed. An association between virologic response at 48 weeks (HIV-1 RNA level <400 copies/mL) and PI-resistance mutations detected in baseline HIV-1 isolates from PI-experienced patients receiving LEXIVA/ritonavir twice daily (n = 88), or lopinavir/ritonavir twice daily (n = 85) in study APV30003 is shown in Table 1. The majority of subjects had previously received either one (47%) or 2 PIs (36%), most commonly nelfinavir (57%) and indinavir (53%). Out of 102 subjects with baseline phenotypes receiving twice-daily LEXIVA/ritonavir, 54% (n = 55) had resistance to at least one PI, with 98% (n = 54) of those having resistance to nelfinavir. Out of 97 subjects with baseline phenotypes in the lopinavir/ritonavir arm, 60% (n = 58) had resistance to at least one PI, with 97% (n = 56) of those having resistance to nelfinavir.
|
PI-mutations† |
LEXIVA/Ritonavir b.i.d. (n = 88) |
Lopinavir/Ritonavir b.i.d. (n = 85) |
||
|
D30N |
21/22 |
95% |
17/19 |
89% |
|
N88D/S |
20/22 |
91% |
12/12 |
100% |
|
L90M |
16/31 |
52% |
17/29 |
59% |
|
M46I/L |
11/22 |
50% |
12/24 |
50% |
|
V82A/F/T/S |
2/9 |
22% |
6/17 |
35% |
|
I54V |
2/11 |
18% |
6/11 |
55% |
|
I84V |
1/6 |
17% |
2/5 |
40% |
*Results should be interpreted with caution because the subgroups were small.
†Most patients had >1 PI resistance-associated mutation at baseline.
The virologic response based upon baseline phenotype was assessed. Baseline isolates from PI-experienced patients responding to LEXIVA/ritonavir twice daily had a median shift in susceptibility to amprenavir relative to a standard wild-type reference strain of 0.7 (range: 0.1 to 5.4, n = 62), and baseline isolates from individuals failing therapy had a median shift in susceptibility of 1.9 (range: 0.2 to 14, n = 29). Because this was a select patient population, these data do not constitute definitive clinical susceptibility break points. Additional data are needed to determine clinically relevant break points for LEXIVA.
Isolates from 15 of the 20 patients receiving twice-daily LEXIVA/ritonavir up to Week 48 and experiencing virologic failure/ongoing replication were subjected to genotypic analysis. The following amprenavir resistance-associated mutations were found either alone or in combination: V32I, M46I/L, I47V, I50V, I54L/M, and I84V. Isolates from 4 of the 16 patients continuing to receive twice-daily LEXIVA/ritonavir up to Week 96 who experienced virologic failure underwent genotypic analysis. Isolates from 2 patients contained amprenavir resistance-associated mutations: V32I, M46I, and I47V in 1 isolate and I84V in the other.
CLINICAL PHARMACOLOGY
Pharmacokinetics in Adults
Fosamprenavir is a prodrug, which is rapidly hydrolyzed to amprenavir by enzymes in the gut epithelium as it is absorbed.
The pharmacokinetic properties of amprenavir after administration of LEXIVA, with or without ritonavir, have been evaluated in both healthy adult volunteers and in HIV-infected patients; no substantial differences in steady-state amprenavir concentrations were observed between the 2 populations.
Absorption and Bioavailability
After administration of a single dose of LEXIVA to HIV−1-infected patients, the time to peak amprenavir concentration (Tmax) occurred between 1.5 and 4 hours (median 2.5 hours). The absolute oral bioavailability of amprenavir after administration of LEXIVA in humans has not been established.
The pharmacokinetic parameters of amprenavir after administration of LEXIVA (with and without concomitant ritonavir) are shown in Table 2.
|
Regimen |
Cmax (mcg/mL) |
Tmax (hours)* |
AUC24 (mcg•hr/mL) |
Cmin (mcg/mL) |
|
LEXIVA 1,400 mg b.i.d. |
4.82 (4.06-5.72) |
1.3 (0.8-4.0) |
33.0 (27.6-39.2) |
0.35 (0.27-0.46) |
|
LEXIVA 1,400 mg q.d. plus Ritonavir 200 mg q.d. |
7.24 (6.32-8.28) |
2.1 (0.8-5.0) |
69.4 (59.7-80.8) |
1.45 (1.16-1.81) |
|
LEXIVA 700 mg b.i.d. plus Ritonavir 100 mg b.i.d. |
6.08 (5.38-6.86) |
1.5 (0.75-5.0) |
79.2 (69.0-90.6) |
2.12 (1.77-2.54) |
*Data shown are median (range).
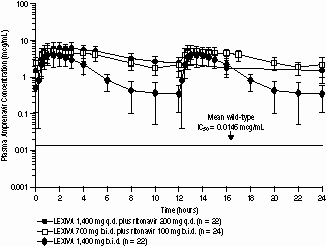
The median plasma amprenavir concentrations of the dosing regimens over the dosing intervals are displayed in Figure 1.
Figure 1. Mean (±SD) Steady-State Plasma Amprenavir Concentrations and Mean IC50 Values Against HIV from Protease Inhibitor-Naive Patients (in the Absence of Human Serum)
Effects of Food on Oral Absorption
LEXIVA Tablets may be taken with or without food (see DOSAGE AND ADMINISTRATION). Administration of a single 1,400-mg dose of LEXIVA in the fed state (standardized high-fat meal: 967 kcal, 67 grams fat, 33 grams protein, 58 grams carbohydrate) compared to the fasted state was associated with no significant changes in amprenavir Cmax, Tmax, or AUC0-∞.
Distribution
In vitro, amprenavir is approximately 90% bound to plasma proteins, primarily to alpha1-acid glycoprotein. In vitro, concentration-dependent binding was observed over the concentration range of 1 to 10 mcg/mL, with decreased binding at higher concentrations.The partitioning of amprenavir into erythrocytes is low, but increases as amprenavir concentrations increase, reflecting the higher amount of unbound drug at higher concentrations.
Metabolism
After oral administration, fosamprenavir is rapidly and almost completely hydrolyzed to amprenavir and inorganic phosphate prior to reaching the systemic circulation. This occurs in the gut epithelium during absorption. Amprenavir is metabolized in the liver by the cytochrome P450 3A4 (CYP3A4) enzyme system. The 2 major metabolites result from oxidation of the tetrahydrofuran and aniline moieties. Glucuronide conjugates of oxidized metabolites have been identified as minor metabolites in urine and feces.
Elimination
Excretion of unchanged amprenavir in urine and feces is minimal. Unchanged amprenavir in urine accounts for approximately 1% of the dose; unchanged amprenavir was not detectable in feces. Approximately 14% and 75% of an administered single dose of 14C-amprenavir can be accounted for as metabolites in urine and feces, respectively. Two metabolites accounted for >90% of the radiocarbon in fecal samples. The plasma elimination half-life of amprenavir is approximately 7.7 hours.
Special Populations
Hepatic Insufficiency
The pharmacokinetics of amprenavir after administration of LEXIVA have not been studied in patients with hepatic insufficiency.
The pharmacokinetics of amprenavir have been studied after administration of amprenavir given as AGENERASE® Capsules to adult patients with impaired hepatic function using a single 600-mg oral dose. The AUC0-∞ of amprenavir was significantly greater in patients with moderate cirrhosis (25.76 ± 14.68 mcg•hr/mL) compared with healthy volunteers (12.00 ± 4.38 mcg•hr/mL). The AUC0-∞ and Cmax were significantly greater in patients with severe cirrhosis (AUC0-∞: 38.66 ± 16.08 mcg•hr/mL; Cmax: 9.43 ± 2.61 mcg/mL) compared with healthy volunteers (AUC0-∞: 12.00 ± 4.38 mcg•hr/mL; Cmax: 4.90 ± 1.39 mcg/mL). Based on these data, patients with impaired hepatic function receiving LEXIVA without concurrent ritonavir may require dosage reduction. There are no data on the use of LEXIVA in combination with ritonavir in patients with any degree of hepatic impairment (see PRECAUTIONS and DOSAGE AND ADMINISTRATION).
Renal Insufficiency
The impact of renal impairment on amprenavir elimination in adult patients has not been studied. The renal elimination of unchanged amprenavir represents approximately 1% of the administered dose; therefore, renal impairment is not expected to significantly impact the elimination of amprenavir.
Pediatric Patients
The pharmacokinetics of amprenavir after administration of LEXIVA to pediatric patients are under investigation. There are insufficient data at this time to recommend a dose.
Geriatric Patients
The pharmacokinetics of amprenavir after administration of LEXIVA to patients over 65 years of age have not been studied.
Gender
The pharmacokinetics of amprenavir after administration of LEXIVA do not differ between males and females.
Race
The pharmacokinetics of amprenavir after administration of LEXIVA do not differ between blacks and non-blacks.
Drug Interactions
See also CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS: Drug Interactions.
Amprenavir, the active metabolite of fosamprenavir, is metabolized in the liver by the cytochrome P450 enzyme system. Amprenavir inhibits CYP3A4. Data also suggestthat amprenavir induces CYP3A4. Caution should be used when coadministering medications that are substrates, inhibitors, or inducers of CYP3A4, or potentially toxic medications that are metabolized by CYP3A4. Amprenavir does not inhibit CYP2D6, CYP1A2, CYP2C9, CYP2C19, CYP2E1, or uridine glucuronosyltransferase (UDPGT).
Drug interaction studies were performed with LEXIVA and other drugs likely to be coadministered or drugs commonly used as probes for pharmacokinetic interactions. The effects of coadministration on AUC, Cmax, and Cmin values are summarized in Table 3 (effect of other drugs on amprenavir) and Table 5 (effect of LEXIVA on other drugs). In addition, since LEXIVA delivers comparable amprenavir plasma concentrations as AGENERASE, drug interaction data derived from studies with AGENERASE are provided in Tables 4 and 6. For information regarding clinical recommendations, see PRECAUTIONS: Drug Interactions.
|
Coadministered Drug(s) and Dose(s) |
Dose of LEXIVA* |
n |
% Change in Amprenavir Pharmacokinetic Parameters (90% CI) |
||
|
Cmax |
AUC |
Cmin |
|||
|
Antacid (MAALOX TC®) 30 mL single dose |
1,400 mg single dose |
30 |
↓35 (↓24 to ↓42) |
↓18 (↓9 to ↓26) |
↑14 (↓7 to ↑39) |
|
Atazanavir 300 mg q.d. for 10 days |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 10 days |
22 |
↔ |
↔ |
↔ |
|
Atorvastatin 10 mg q.d. for 4 days |
1,400 mg b.i.d. for 2 weeks |
16 |
↓18 (↓34 to ↑1) |
↓27 (↓41 to ↓12) |
↓12 (↓27 to ↓6) |
|
Atorvastatin 10 mg q.d. for 4 days |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 2 weeks |
16 |
↔ |
↔ |
↔ |
|
Efavirenz 600 mg q.d. for 2 weeks |
1,400 mg q.d. plus ritonavir 200 mg q.d. for 2 weeks |
16 |
↔ |
↓13 (↓30 to ↑7) |
↓36 (↓8 to ↓56) |
|
Efavirenz 600 mg q.d. plus additional ritonavir 100 mg q.d. for 2 weeks |
1,400 mg q.d. plus ritonavir 200 mg q.d. for 2 weeks |
16 |
↑18 (↑1 to ↑38) |
↑11 (0 to ↑24) |
↔ |
|
Efavirenz 600 mg q.d. for 2 weeks |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 2 weeks |
16 |
↔ |
↔ |
↓17 (↓4 to ↓29) |
|
Esomeprazole 20 mg q.d. for 2 weeks |
1,400 mg b.i.d. for 2 weeks |
25 |
↔ |
↔ |
↔ |
|
Esomeprazole 20 mg q.d. for 2 weeks |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 2 weeks |
23 |
↔ |
↔ |
↔ |
|
Ethinyl estradiol/norethindrone 0.035 mg/0.5 mg q.d. for 21 days |
700 mg b.i.d. plus ritonavir† 100 mg b.i.d. for 21 days |
25 |
↔‡ |
↔‡ |
↔‡ |
|
Ketoconazole§ 200 mg q.d. for 4 days |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 4 days |
15 |
↔ |
↔ |
↔ |
|
Lopinavir/ritonavir 533 mg/133 mg b.i.d. |
1,400 mg b.i.d. for 2 weeks |
18 |
See following section: HIV Protease Inhibitors |
||
|
Lopinavir/ritonavir 400 mg/100 mg b.i.d. for 2 weeks |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 2 weeks |
18 |
↓58 (↓42 to ↓70) |
↓63 (↓51 to ↓72) |
↓65 (↓54 to ↓73) |
|
Nevirapine 200 mg b.i.d. for 2 weeks║ |
1,400 mg b.i.d. for 2 weeks |
17 |
↓25 (↓37 to ↓10) |
↓33 (↓45 to ↓20) |
↓35 (↓50 to ↓15) |
|
Nevirapine 200 mg b.i.d. for 2 weeks║ |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 2 weeks |
17 |
↔ |
↓11 (↓23 to ↑3) |
↓19 (↓32 to ↓4) |
|
Ranitidine 300 mg single dose (administered 1 hour before fosamprenavir) |
1,400 mg single dose |
30 |
↓51 (↓43 to ↓58) |
↓30 (↓22 to ↓37) |
↔ (↓19 to ↑21) |
|
Rifabutin 150 mg q.o.d.for 2 weeks |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 2 weeks |
15 |
↑36‡ (↑18 to ↑55) |
↑35‡ (↑17 to ↑56) |
↑17‡ (↓1 to ↑39) |
* Concomitant medication is also shown in this column where appropriate.
†Ritonavir Cmax, AUC, and Cmin increased by 63%, 45%, and 13%, respectively, compared to historical control.
‡Compared to historical control.
§Patients were receiving LEXIVA/ritonavir for 10 days prior to the 4-day treatment period with both ketoconazole and LEXIVA/ritonavir.
║Patients were receiving nevirapine for at least 12 weeks prior to study.
↑= Increase; ↓= Decrease; ↔ = No change (↑or ↓≤10%).
|
Coadministered Drug(s) and Dose(s) |
Dose of AGENERASE* |
n |
% Change in Amprenavir Pharmacokinetic Parameters (90% CI) |
||
|
Cmax |
AUC |
Cmin |
|||
|
Clarithromycin 500 mg b.i.d. for 4 days |
1,200 mg b.i.d. for 4 days |
12 |
↑15 (↑1 to ↑31) |
↑18 (↑8 to ↑29) |
↑39 (↑31 to ↑47) |
|
Delavirdine 600 mg b.i.d. for 10 days |
600 mg b.i.d. for 10 days |
9 |
↑40* |
↑130* |
↑125* |
|
Ethinyl estradiol/norethindrone 0.035 mg/1 mg for 1 cycle |
1,200 mg b.i.d. for 28 days |
10 |
↔ |
↓22 (↓35 to ↓8) |
↓20 ↓41 to ↑8) |
|
Indinavir 800 mg t.i.d. for 2 weeks (fasted) |
750 or 800 mg t.i.d. for 2 weeks (fasted) |
9 |
↑18 (↓13 to ↑58) |
↑33 (↑2 to ↑73) |
↑25 (↓27 to ↑116) |
|
Ketoconazole 400 mg single dose |
1,200 mg single dose |
12 |
↓16 (↓25 to ↓6) |
↑31 (↑20 to ↑42) |
NA |
|
Lamivudine 150 mg single dose |
600 mg single dose |
11 |
↔ |
↔ |
NA |
|
Nelfinavir 750 mg t.i.d. for 2 weeks (fed) |
750 or 800 mg t.i.d. for 2 weeks (fed) |
6 |
↓14 (↓38 to ↑20) |
↔ |
↑189 (↑52 to ↑448) |
|
Rifabutin 300 mg q.d. for 10 days |
1,200 mg b.i.d. for 10 days |
5 |
⇔ |
↓15 (↓28 to 0) |
↓15 (↓38 to ↑17) |
|
Rifampin 300 mg q.d. for 4 days |
1,200 mg b.i.d. for 4 days |
11 |
↓70 (↓76 to ↓62) |
↓82 (↓84 to ↓78) |
↓92 (↓95 to ↓89) |
|
Saquinavir 800 mg t.i.d. for 2 weeks (fed) |
750 or 800 mg t.i.d. for 2 weeks (fed) |
7 |
↓37 (↓54 to ↓14) |
↓32 (↓49 to ↓9) |
↓14 (↓52 to ↑54) |
|
Zidovudine 300 mg single dose |
600 mg single dose |
12 |
↔ |
↑13 (↓2 to ↑31) |
NA |
*Median percent change; confidence interval not reported.
↑= Increase; ↓= Decrease; ↔ = No change (↑or ↓<10%); NA = Cmin not calculated for single-dose study.
|
Coadministered Drug(s) and Dose(s) |
Dose of LEXIVA* |
n |
% Change inPharmacokinetic Parameters of Coadministered Drug (90% CI) |
||
|
Cmax |
AUC |
Cmin |
|||
|
Atazanavir 300 mg q.d. for 10 days† |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 10 days |
21 |
↓24 (↓39 to ↓6) |
↓22 (↓34 to ↓9) |
↔ |
|
Atorvastatin 10 mg q.d. for 4 days |
1,400 mg b.i.d. for 2 weeks |
16 |
↑304 (↑205 to ↑437) |
↑130 (↑100 to ↑164) |
↓10 (↓27 to ↑12) |
|
Atorvastatin 10 mg q.d. for 4 days |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 2 weeks |
16 |
↑184 (↑126 to ↑257) |
↑153 (↑115 to ↑199) |
↑73 (↑45 to ↑108) |
|
Esomeprazole 20 mg q.d. for 2 weeks |
1,400 mg b.i.d. for 2 weeks |
25 |
↔ |
↑55 (↑39 to ↑73) |
ND |
|
Esomeprazole 20 mg q.d. for 2 weeks |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 2 weeks |
23 |
↔ |
↔ |
ND |
|
Ethinyl estradiol‡ 0.035 mg q.d. for 21 days |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 21 days |
25 |
↓28 (↓21 to ↓35 |
↓37 (↓30 to ↓42) |
ND |
|
Ketoconazole§ 200 mg q.d. for 4 days |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 4 days |
15 |
↑25 (↑0 to ↑56) |
↑169 (↑108 to ↑248) |
ND |
|
Lopinavir/ritonavir║ 533 mg/133 mg b.i.d. for 2 weeks |
1,400 mg b.i.d. for 2 weeks |
18 |
See following section: HIV Protease Inhibitors |
||
|
Lopinavir/ritonavir║ 400 mg/100 mg b.i.d. for 2 weeks |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 2 weeks |
18 |
↑30 (↓15 to ↑47) |
↑37 (↓20 to ↑55) |
↑52 (↓28 to ↑82) |
|
Nevirapine 200 mg b.i.d. for 2 weeks¶ |
1,400 mg b.i.d. for 2 weeks |
17 |
↑25 (↑14 to ↑37) |
↑29 (↑19 to ↑40) |
↑34 (↑20 to ↑49) |
|
Nevirapine 200 mg b.i.d. for 2 weeks¶ |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 2 weeks |
17 |
↑13 (↑3 to ↑24) |
↑14 (↑5 to ↑24) |
↑22 (↑9 to ↑35) |
|
Norethindrone‡ 0.5 mg q.d.for 21 days |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 21 days |
25 |
↓38 (↓32 to ↓44) |
↓34 (↓30 to↓37) |
↓26 (↓20 to ↓32) |
|
Rifabutin 150 mg every other day for 2 weeks # (25-O-desacetylrifabutin metabolite) Rifabutin + 25-O- desacetylrifabutin metabolite |
700 mg b.i.d. plus ritonavir 100 mg b.i.d. for 2 weeks |
15 |
↓14 (↓28 to ↑4) ↑579 (↑479 to ↑698) NA |
↔ ↑1,120 (↑965 to ↑1,300) ↑64 (↑46 to ↑84) |
↑28 (↑12 to ↑46) ↑2,510 (↑1,910 to ↑3,300) NA |
* Concomitant medication is also shown in this column where appropriate.
†Comparison arm of atazanavir 300 mg q.d. plus ritonavir 100 mg q.d. for 10 days.
‡ Administered as a combination oral contraceptive tablet: ethinyl estradial 0.035 mg/norethindrone 0.5 mg.
§Patients were receiving LEXIVA/ritonavir for 10 days prior to the 4-day treatment period with both ketoconazole and LEXIVA/ritonavir.
║Data represent lopinavir concentrations.
¶Patients were receiving nevirapine for at least 12 weeks prior to study.
# Comparison arm of rifabutin 300 mg q.d. for 2 weeks. AUC is AUC(0-48 hr).
↑= Increase; ↓= Decrease; ↔ = No change (↑or ↓<10%); ND = Interaction cannot be determined as Cmin was below the lower limit of quantitation.
|
Coadministered Drug(s) and Dose(s) |
Dose of AGENERASE |
n |
% Change inPharmacokinetic Parameters of Coadministered Drug (90% CI) |
||
|
Cmax |
AUC |
Cmin |
|||
|
Clarithromycin 500 mg b.i.d. for 4 days |
1,200 mg b.i.d. for 4 days |
12 |
↓10 (↓24 to ↑7) |
↔ |
↔ |
|
Delavirdine 600 mg b.i.d. for 10 days |
600 mg b.i.d. for 10 days |
9 |
↓47* |
↓61* |
↓88* |
|
Ethinyl estradiol 0.035 mg for 1 cycle |
1,200 mg b.i.d. for 28 days |
10 |
↔ |
↔ |
↑32 (↓3 to ↑79) |
|
Ketoconazole 400 mg single dose |
1,200 mg single dose |
12 |
↑19 (↑8 to ↑33) |
↑44 (↑31 to ↑59) |
NA |
|
Lamivudine 150 mg single dose |
600 mg single dose |
11 |
↔ |
↔ |
NA |
|
Methadone 44 to 100 mg q.d. for >30 days |
1,200 mg b.i.d. for 10 days |
16 |
R-Methadone (active) |
||
|
↓25 (↓32 to ↓18) |
↓13 (↓21 to ↓5) |
↓21 (↓32 to ↓9) |
|||
|
S-Methadone (inactive) |
|||||
|
↓48 (↓55 to ↓40) |
↓40 (↓46 to ↓32) |
↓53 (↓60 to ↓43) |
|||
|
Norethindrone 1 mg for 1 cycle |
1,200 mg b.i.d. for 28 days |
10 |
↔ |
↑18 ↑1 to ↑38 |
↑45 ↑13 to ↑88 |
|
Rifabutin 300 mg q.d. for 10 days |
1,200 mg b.i.d. for 10 days |
5 |
↑119 (↑82 to ↑164) |
↑193 (↑156 to ↑235) |
↑271 (↑171 to ↑409) |
|
Rifampin 300 mg q.d. for 4 days |
1,200 mg b.i.d. for 4 days |
11 |
↔ |
↔ |
ND |
|
Zidovudine 300 mg single dose |
600 mg single dose |
12 |
↑40 (↑14 to ↑71) |
↑31 (↑19 to ↑45) |
NA |
*Median percent change; confidence interval not reported.
↑= Increase; ↓= Decrease; ↔ = No change (↑or ↓<10%); NA = Cmin not calculated for single-dose study; ND = Interaction cannot be determined as Cmin was below the lower limit of quantitation.
Nucleoside Reverse Transcriptase Inhibitors
There was no clinically significant effect of amprenavir after administration of AGENERASE on abacavir in subjects receiving both agents based on historical data.
In a Phase III clinical trial (APV30003), plasma amprenavir trough concentrations were similar for subjects receiving tenofovir disoproxil fumarate in combination with LEXIVA and ritonavir as compared to subjects not receiving tenofovir.
HIV Protease Inhibitors
In a 3-arm, randomized, cross-over study involving healthy volunteers, amprenavir pharmacokinetics were compared after administration of LEXIVA 1,400 mg twice daily plus lopinavir/ritonavir 533 mg/133 mg twice daily for 2 weeks versus LEXIVA 700 mg twice daily plus ritonavir 100 mg twice daily for 2 weeks. Amprenavir concentrations were lower with the regimen containing lopinavir/ritonavir: Cmax was 13% lower, AUC was 26% lower, and Cmin was 42% lower. In the same study, lopinavir pharmacokinetics were compared after administration of LEXIVA 1,400 mg twice daily plus lopinavir/ritonavir 533 mg/133 mg twice daily for 2 weeks versus lopinavir/ritonavir 400 mg/100 mg twice daily for 2 weeks. Lopinavir concentrations were similar (less than 10% change in Cmax, AUC, and Cmin values) with these 2 regimens.
The effect of amprenavir after administration of AGENERASE Capsules on concentrations of other HIV protease inhibitors in subjects receiving both agents was evaluated using comparisons to historical data. Indinavir steady-state Cmax, AUC, and Cmin were decreased by 22%, 38%, and 27%, respectively, by concomitant amprenavir. Similar decreases in Cmax and AUC were seen after the first dose. Saquinavir steady-state Cmax, AUC, and Cmin were increased 21%, decreased 19%, and decreased 48%, respectively, by concomitant amprenavir. Nelfinavir steady-state Cmax, AUC, and Cmin were increased by 12%, 15%, and 14%, respectively, by concomitant amprenavir.
Methadone
Coadministration of amprenavir and methadone can decrease plasma levels of methadone.
Coadministration of amprenavir and methadone as compared to a non-matched historical control group resulted in a 30%, 27%, and 25% decrease in serum amprenavir AUC, Cmax, and Cmin, respectively.
INDICATIONS AND USAGE
LEXIVA is indicated in combination with other antiretroviral agents for the treatment of HIV infection in adults.
The following points should be considered when initiating therapy with LEXIVA/ritonavir in protease inhibitor-experienced patients (see Description of Clinical Studies).
- The protease inhibitor-experienced patient study was not large enough to reach a definitive conclusion that LEXIVA/ritonavir and lopinavir/ritonavir are clinically equivalent.
- Once-daily administration of LEXIVA plus ritonavir is not recommended for protease inhibitor-experienced patients.
Description of Clinical Studies
Therapy-Naive Patients
Study APV30001
APV30001 was a randomized, open-label study, comparing treatment with LEXIVA Tablets (1,400 mg twice daily) versus nelfinavir (1,250 mg twice daily) in 249 antiretroviral treatment-naive patients. Both groups of patients also received abacavir (300 mg twice daily) and lamivudine (150 mg twice daily).
The mean age of the patients in this study was 37 years (range 17 to 70 years), 69% of the patients were males, 20% were CDC Class C (AIDS), 24% were Caucasian, 32% were black, and 44% were Hispanic. At baseline, the median CD4+ cell count was 212 cells/mm3 (range: 2 to 1,136 cells/mm3; 18% of patients had a CD4+ cell count of <50 cells/mm3 and 30% were in the range of 50 to <200 cells/mm3). Baseline median HIV-1 RNA was 4.83 log10 copies/mL (range: 1.69 to 7.41 log10 copies/mL; 45% of patients had >100,000 copies/mL).
The outcomes of randomized treatment are provided in Table 7.
|
Outcome (Rebound or discontinuation = failure) |
LEXIVA 1,400 mg b.i.d. (n = 166) |
Nelfinavir 1,250 mg b.i.d. (n = 83) |
|
Responder* |
66% (57%) |
52% (42%) |
|
Virologic failure |
19% |
32% |
|
Rebound |
16% |
19% |
|
Never suppressed through Week 48 |
3% |
13% |
|
Clinical progression |
1% |
1% |
|
Death |
0% |
1% |
|
Discontinued due to adverse reactions |
4% |
2% |
|
Discontinued due to other reasons† |
10% |
10% |
*Patients achieved and maintained confirmed HIV-1 RNA <400 copies/mL (<50 copies/mL) through Week 48 (Roche AMPLICOR HIV-1 MONITOR Assay Version 1.5).
†Includes consent withdrawn, lost to follow up, protocol violations, those with missing data, and other reasons.
Treatment response by viral load strata is shown in Table 8.
|
Screening Viral Load HIV-1 RNA (copies/mL) |
LEXIVA 1,400 mg b.i.d. |
Nelfinavir 1,250 mg b.i.d. |
||
|
<400 copies/mL |
n |
<400 copies/mL |
N |
|
|
≤100,000 |
65% |
93 |
65% |
46 |
|
>100,000 |
67% |
73 |
36% |
37 |
Through 48 weeks of therapy, the median increases from baseline in CD4+ cell counts were 201 cells/mm3 in the group receiving LEXIVA and 216 cells/mm3 in the nelfinavir group.
Study APV30002
APV30002 was a randomized, open-label study, comparing treatment with LEXIVA Tablets (1,400 mg once daily) plus ritonavir (200 mg once daily) versus nelfinavir (1,250 mg twice daily) in 649 treatment-naive patients. Both treatment groups also received abacavir (300 mg twice daily) and lamivudine (150 mg twice daily).
The mean age of the patients in this study was 37 years (range 18 to 69 years), 73% of the patients were males, 22% were CDC Class C, 53% were Caucasian, 36% were black, and 8% were Hispanic. At baseline, the median CD4+ cell count was 170 cells/mm3 (range: 1 to 1,055 cells/mm3; 20% of patients had a CD4+ cell count of <50 cells/mm3 and 35% were in the range of 50 to <200 cells/mm3). Baseline median HIV-1 RNA was 4.81 log10 copies/mL (range: 2.65 to 7.29 log10 copies/mL; 43% of patients had >100,000 copies/mL).
The outcomes of randomized treatment are provided in Table 9.
|
Outcome (Rebound or discontinuation = failure) |
LEXIVA 1,400 mg q.d./ Ritonavir 200 mg q.d. (n = 322) |
Nelfinavir 1,250 mg b.i.d. (n = 327) |
|
Responder* |
69% (58%) |
68% (55%) |
|
Virologic failure |
6% |
16% |
|
Rebound |
5% |
8% |
|
Never suppressed through Week 48 |
1% |
8% |
|
Death |
1% |
0% |
|
Discontinued due to adverse reactions |
9% |
6% |
|
Discontinued due to other reasons† |
15% |
10% |
*Patients achieved and maintained confirmed HIV-1 RNA <400 copies/mL (<50 copies/mL) through Week 48 (Roche AMPLICOR HIV-1 MONITOR Assay Version 1.5).
†Includes consent withdrawn, lost to follow up, protocol violations, those with missing data, and other reasons.
Treatment response by viral load strata is shown in Table 10.
|
Screening Viral Load HIV-1 RNA |
LEXIVA 1,400 mg q.d./Ritonavir 200 mg q.d. |
Nelfinavir 1,250 mg b.i.d. |
||
|
(copies/mL) |
<400 copies/mL |
n |
<400 copies/mL |
N |
|
≤100,000 |
72% |
197 |
73% |
194 |
|
>100,000 |
66% |
125 |
64% |
133 |
Through 48 weeks of therapy, the median increases from baseline in CD4+ cell counts were 203 cells/mm3 in the group receiving LEXIVA and 207 cells/mm3 in the nelfinavir group.
Protease Inhibitor-Experienced Patients
Study APV30003
APV30003 was a randomized, open-label, multicenter study comparing 2 different regimens of LEXIVA plus ritonavir (LEXIVA Tablets 700 mg twice daily plus ritonavir 100 mg twice daily or LEXIVA Tablets 1,400 mg once daily plus ritonavir 200 mg once daily) versus lopinavir/ritonavir (400 mg/100 mg twice daily) in 315 patients who had experienced virologic failure to 1 or 2 prior protease inhibitor-containing regimens.
The mean age of the patients in this study was 42 years (range 24 to 72 years), 85% were male, 33% were CDC Class C, 67% were Caucasian, 24% were black, and 9% were Hispanic. The median CD4+ cell count at baseline was 263 cells/mm3 (range: 2 to 1,171 cells/mm3). Baseline median plasma HIV-1 RNA level was 4.14 log10 copies/mL (range: 1.69 to 6.41 log10 copies/mL).
The median durations of prior exposure to NRTIs were 257 weeks for patients receiving LEXIVA/ritonavir twice daily (79% had ≥3 prior NRTIs) and 210 weeks for patients receiving lopinavir/ritonavir (64% had ≥3 prior NRTIs). The median durations of prior exposure to protease inhibitors were 149 weeks for patients receiving LEXIVA/ritonavir twice daily (49% received ≥2 prior PIs) and 130 weeks for patients receiving lopinavir/ritonavir (40% received ≥2 prior PIs).
The time-averaged changes in plasma HIV-1 RNA from baseline (AAUCMB) at 48 weeks (the endpoint on which the study was powered) were -1.4 log10 copies/mL for twice-daily LEXIVA/ritonavir and -1.67 log10 copies/mL for the lopinavir/ritonavir group.
The proportions of patients who achieved and maintained confirmed HIV-1 RNA <400 copies/mL (secondary efficacy endpoint) were 58% with twice-daily LEXIVA/ritonavir and 61% with lopinavir/ritonavir (95% CI for the difference -16.6, 10.1). The proportions of patients with HIV-1 RNA <50 copies/mL with twice-daily LEXIVA/ritonavir and with lopinavir/ritonavir were 46% and 50%, respectively (95% CI for the difference -18.3, 8.9). The proportions of patients who were virologic failures were 29% with twice-daily LEXIVA/ritonavir and 27% with lopinavir/ritonavir.
The frequency of discontinuations due to adverse events and other reasons, and deaths were similar between treatment arms.
Through 48 weeks of therapy, the median increases from baseline in CD4+ cell counts were 81 cells/mm3 with twice-daily LEXIVA/ritonavir and 91 cells/mm3 with lopinavir/ritonavir.
This study was not large enough to reach a definitive conclusion that LEXIVA/ritonavir and lopinavir/ritonavir are clinically equivalent.
Once-daily administration of LEXIVA plus ritonavir is not recommended for protease inhibitor-experienced patients. Through Week 48, 50% and 37% of patients receiving LEXIVA/ritonavir once daily had plasma HIV-1 RNA <400 copies/mL and <50 copies/mL, respectively.
CONTRAINDICATIONS
LEXIVAis contraindicated in patients with previously demonstrated clinically significant hypersensitivity to any of the components of this product or to amprenavir.
Coadministration of LEXIVA with drugs that are highly dependent on CYP3A4 for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events is contraindicated. These drugs are listed in Table 11.
|
Drug Class |
Drugs Within Class That Are CONTRAINDICATED with LEXIVA |
|
Ergot derivatives |
Dihydroergotamine, ergonovine, ergotamine, methylergonovine |
|
GI motility agent |
Cisapride |
|
Neuroleptic |
Pimozide |
|
Sedatives/hypnotics |
Midazolam, triazolam |
If LEXIVA is coadministered with ritonavir, the antiarrhythmic agents flecainide and propafenone are also contraindicated. Also, refer to the full prescribing information for NORVIR® (ritonavir) for other potential drug interactions.
WARNINGS
Serious and/or life-threatening drug interactions could occur between LEXIVA and amiodarone, lidocaine (systemic), tricyclic antidepressants, and quinidine. Concentration monitoring of these agents is recommended if these agents are used concomitantly with LEXIVA (see CONTRAINDICATIONS).
Severe and life-threatening skin reactions, including Stevens-Johnson syndrome, have occurred in patients treated with amprenavir (see ADVERSE REACTIONS). Acute hemolytic anemia has been reported in a patient treated with amprenavir.
Rifampin should not be used in combination with LEXIVA because it reduces plasma concentrations of amprenavir by about 90%. The effect of rifampin on amprenavir concentrations when rifampin is administered with LEXIVA plus ritonavir is not known.
A drug interaction study in healthy subjects has shown that ritonavir significantly increases plasma fluticasone propionate exposures, resulting in significantly decreased serum cortisol concentrations. Concomitant use of LEXIVA with ritonavir and fluticasone propionate is expected to produce the same effects. Systemic corticosteroid effects including Cushing’s syndrome and adrenal suppression have been reported during postmarketing use in patients receiving ritonavir and inhaled or intranasally administered fluticasone propionate. Therefore, the coadministration of fluticasone propionate and LEXIVA/ritonavir is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects (see PRECAUTIONS: Drug Interactions).
Concomitant use of LEXIVA and St. John's wort (hypericum perforatum) or products containing St. John's wort is not recommended. Coadministration of protease inhibitors, including LEXIVA, with St. John's wort is expected to substantially decrease protease inhibitor concentrations and may result in suboptimal levels of amprenavir and lead to loss of virologic response and possible resistance to LEXIVA or to the class of protease inhibitors.
Concomitant use of LEXIVA with lovastatin or simvastatin is not recommended. Caution should be exercised if HIV protease inhibitors, including LEXIVA, are used concurrently with other HMG-CoA reductase inhibitors that are also metabolized by the CYP3A4 pathway (e.g., atorvastatin). The risk of myopathy, including rhabdomyolysis, may be increased when HIV protease inhibitors, including LEXIVA, are used in combination with these drugs.
Particular caution should be used when prescribing phosphodiesterase (PDE5) inhibitors for erectile dysfunction (e.g., sildenafil or vardenafil) in patients receiving protease inhibitors, including LEXIVA. Coadministration of a protease inhibitor with a PDE5 inhibitor is expected to substantially increase the PDE5 inhibitor concentration and may result in an increase in PDE5 inhibitor-associated adverse events, including hypotension, visual changes, and priapism (see PRECAUTIONS: Drug Interactions and Information for Patients, and the complete specific PDE5 inhibitor prescribing information).
Concomitant use of LEXIVA with ritonavir and oral contraceptives is not recommended. LEXIVA with ritonavir and oral contraceptives may result in clinically significant hepatic transaminase elevations. Therefore, alternate methods of non-hormonal contraception are recommended (see PRECAUTIONS: Drug Interactions).
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported during postmarketing surveillance in HIV-infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and causal relationships between protease inhibitor therapy and these events have not been established.
PRECAUTIONS
Sulfa Allergy
LEXIVA should be used with caution in patients with a known sulfonamide allergy. Fosamprenavir contains a sulfonamide moiety. The potential for cross-sensitivity between drugs in the sulfonamide class and fosamprenavir is unknown. In a clinical study of LEXIVA used as the sole protease inhibitor, rash occurred in 2 of 10 patients (20%) with a history of sulfonamide allergy compared with 42 of 126 patients (33%) with no history of sulfonamide allergy. In 2 clinical studies of LEXIVA plus low-dose ritonavir, rash occurred in 8 of 50 patients (16%) with a history of sulfonamide allergy compared with 50 of 412 patients (12%) with no history of sulfonamide allergy.
Hepatic Impairment and Toxicity
LEXIVA is principally metabolized by the liver; therefore, caution should be exercised when administering LEXIVA to patients with hepatic impairment because amprenavir concentrations may be increased (see CLINICAL PHARMACOLOGY: Special Populations: Hepatic Insufficiency). Patients with impaired hepatic function receiving LEXIVA without concurrent ritonavir may require dose reduction (see DOSAGE AND ADMINISTRATION). There are no data on the use of LEXIVA in combination with ritonavir in patients with any degree of hepatic impairment.
Patients with underlying hepatitis B or C or marked elevations in transaminases prior to treatment may be at increased risk for developing transaminase elevations. Appropriate laboratory testing should be conducted prior to initiating therapy with LEXIVA and patients should be monitored closely during treatment.
Use of LEXIVA with ritonavir at higher-than-recommended dosages may result in transaminase elevations and should not be used (see OVERDOSAGE and DOSAGE AND ADMINISTRATION).
Patients With Hemophilia
There have been reports of spontaneous bleeding in patients with hemophilia A and B treated with protease inhibitors. In some patients, additional factor VIII was required. In many of the reported cases, treatment with protease inhibitors was continued or restarted. A causal relationship between protease inhibitor therapy and these episodes has not been established.
Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including LEXIVA. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Fat Redistribution
Redistribution/accumulation of body fat, including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance,” have been observed in patients receiving antiretroviral therapy, including LEXIVA. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
Lipid Elevations
Treatment with LEXIVA plus ritonavir has resulted in increases in the concentration of triglycerides (see Tables 16 and 17). Triglyceride and cholesterol testing should be performed prior to initiating therapy with LEXIVA and at periodic intervals during therapy. Lipid disorders should be managed as clinically appropriate. (See PRECAUTIONS: Table 12: Drugs That Should Not Be Coadministered with LEXIVA and Table 13: Established and Other Potentially Significant Drug Interactionsfor additional information on potential drug interactions with LEXIVA and HMG-CoA reductase inhibitors.)
Resistance/Cross-Resistance
Because the potential for HIV cross-resistance among protease inhibitors has not been fully explored, it is unknown what effect therapy with LEXIVA will have on the activity of subsequently administered protease inhibitors. LEXIVA has been studied in patients who have experienced treatment failure with protease inhibitors (see INDICATIONS AND USAGE: Description of Clinical Studies).
Information for Patients
A statement to patients and healthcare providers is included on the product's bottle label: ALERT: Find out about medicines that should NOT be taken with LEXIVA. A Patient Information Sheet for LEXIVA Tablets is available for patient information.
Patients should be informed that LEXIVA is not a cure for HIV infection and that they may continue to develop opportunistic infections and other complications associated with HIV disease. The long-term effects of LEXIVA are unknown at this time. Patients should be told that there are currently no data demonstrating that therapy with LEXIVA can reduce the risk of transmitting HIV to others.
Patients should be told that sustained decreases in plasma HIV-1 RNA have been associated with a reduced risk of progression to AIDS and death. Patients should remain under the care of a physician while using LEXIVA. Patients should be advised to take LEXIVA every day as prescribed. LEXIVA must always be used in combination with other antiretroviral drugs. Patients should not alter the dose or discontinue therapy without consulting their physician. If a dose is missed, patients should take the dose as soon as possible and then return to their normal schedule. However, if a dose is skipped, the patient should not double the next dose.
Patients should inform their healthcare provider if they have a sulfa allergy. The potential for cross-sensitivity between drugs in the sulfonamide class and fosamprenavir is unknown. LEXIVA may interact with many drugs; therefore, patients should be advised to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products, particularly St. John's wort.
Patients receiving PDE5 inhibitors should be advised that they may be at an increased risk of PDE5 inhibitor-associated adverse events, including hypotension, visual changes, and priapism, and should promptly report any symptoms to their healthcare provider.
Patients receiving hormonal contraceptives should be instructed to use alternate contraceptive measures during therapy with LEXIVA because hormonal levels may be altered, and if used in combination with LEXIVA and ritonavir, liver enzyme elevations may occur.
Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy, including LEXIVA, and that the cause and long-term health effects of these conditions are not known at this time.
Drug Interactions
See also CONTRAINDICATIONS, WARNINGS, and CLINICAL PHARMACOLOGY: Drug Interactions.
Amprenavir, the active metabolite of fosamprenavir, is an inhibitor of cytochrome P450 3A4 metabolism and therefore should not be administered concurrently with medications with narrow therapeutic windows that are substrates of CYP3A4. Data also suggest that amprenavir induces CYP3A4.
Amprenavir is metabolized by CYP3A4. Coadministration of LEXIVA and drugs that induce CYP3A4, such as rifampin, may decrease amprenavir concentrations and reduce its therapeutic effect. Coadministration of LEXIVA and drugs that inhibit CYP3A4 may increase amprenavir concentrations and increase the incidence of adverse effects.
The potential for drug interactions with LEXIVA changes when LEXIVA is coadministered with the potent CYP3A4 inhibitor ritonavir. The magnitude of CYP3A4-mediated drug interactions (effect on amprenavir or effect on coadministered drug) may change when LEXIVA is coadministered with ritonavir. Because ritonavir is a CYP2D6 inhibitor, clinically significant interactions with drugs metabolized by CYP2D6 are possible when coadministered with LEXIVA plus ritonavir.
There are other agents that may result in serious and/or life-threatening drug interactions (see CONTRAINDICATIONS and WARNINGS).
|
Drug Class/Drug Name |
Clinical Comment |
|
Antiarrhythmics: Flecainide, propafenone |
CONTRAINDICATED if LEXIVA is co-prescribed with ritonavir due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias secondary to increases in plasma concentrations of antiarrhythmics. |
|
Antimycobacterials: Rifampin* |
May lead to loss of virologic response and possible resistance to LEXIVA or to the class of protease inhibitors. |
|
Ergot derivatives: Dihydroergotamine, ergonovine, ergotamine, methylergonovine |
CONTRAINDICATED due to potential for serious and/or life-threatening reactions such as acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues. |
|
GI motility agents: Cisapride |
CONTRAINDICATED due topotential for serious and/or life-threatening reactions such as cardiac arrhythmias. |
|
Herbal products: St. John’s wort (hypericum perforatum) |
May lead to loss of virologic response and possible resistance to LEXIVA or to the class of protease inhibitors. |
|
HMG co-reductase inhibitors: Lovastatin, simvastatin |
Potential for serious reactions such as risk of myopathy including rhabdomyolysis. |
|
Neuroleptic: Pimozide |
CONTRAINDICATED due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias. |
|
Non-nucleoside reverse transcriptase inhibitor: Delavirdine* |
May lead to loss of virologic response and possible resistance to delavirdine. |
|
Sedative/hypnotics: Midazolam, triazolam |
CONTRAINDICATED due to potential for serious and/or life-threatening reactions such as prolonged or increased sedation or respiratory depression. |
|
Oral contraceptives: Ethinyl estradiol/norethindrone* |
Alternative methods of non-hormonal contraception are recommended. LEXIVA/ritonavir: Increased risk of transaminase elevations. No data are available on the use of LEXIVA/ritonavir with other hormonal therapies, such as HRT for postmenopausal women (see WARNINGS). LEXIVA without ritonavir: May lead to loss of virologic response (see CLINICAL PHARMACOLOGY, Table 5). |
*See CLINICAL PHARMACOLOGY Tables 3, 4, 5, or 6 for magnitude of interaction.
|
Concomitant Drug Class: Drug Name |
Effect on Concentration of Amprenavir or Concomitant Drug |
Clinical Comment |
|
HIV-Antiviral Agents |
||
|
Non-nucleoside reverse transcriptase inhibitor: Efavirenz* |
LEXIVA: ↓Amprenavir LEXIVA/ritonavir: ↓Amprenavir |
Appropriate doses of the combinations with respect to safety and efficacy have not been established. An additional 100 mg/day (300 mg total) of ritonavir is recommended when efavirenz is administered with LEXIVA/ritonavir once daily. No change in the ritonavir dose is required when efavirenz is administered with LEXIVA plus ritonavir twice daily. |
|
Non-nucleoside reverse transcriptase inhibitor: Nevirapine* |
LEXIVA: ↓Amprenavir ↑Nevirapine LEXIVA/ritonavir: ↓Amprenavir ↑Nevirapine |
Coadministration of nevirapine and LEXIVA without ritonavir is not recommended. No dosage adjustment required when nevirapine is administered with LEXIVA/ritonavir twice daily. The combination of nevirapine administered with LEXIVA/ritonavir once-daily regimen has not been studied. |
|
HIV protease inhibitor: Atazanavir* |
LEXIVA: Interaction has not been evaluated. LEXIVA/ritonavir: ¯Atazanavir «Amprenavir |
Appropriate doses of the combinations with respect to safety and efficacy have not been established. |
|
HIV protease inhibitors: Indinavir*, nelfinavir* |
LEXIVA: ↑Amprenavir Effect on indinavir and nelfinavir is not well established. LEXIVA/ritonavir: Interaction has not been evaluated. |
Appropriate doses of the combinations with respect to safety and efficacy have not been established. |
|
HIV protease inhibitors: Lopinavir/ritonavir* |
↓Amprenavir ↓Lopinavir |
An increased rate of adverse events has been observed with coadministration of these medications. Appropriate doses of the combinations with respect to safety and efficacy have not been established. |
|
HIV protease inhibitor: Saquinavir* |
LEXIVA: ↓Amprenavir Effect on saquinavir is not well established. LEXIVA/ritonavir: Interaction has not been evaluated. |
Appropriate doses of the combination with respect to safety and efficacy have not been established. |
|
Other Agents |
||
|
Antiarrhythmics: Amiodarone, lidocaine (systemic), and quinidine |
↑Antiarrhythmics |
Caution is warranted and therapeutic concentration monitoring, if available, is recommended for antiarrhythmics when coadministered with LEXIVA. |
|
Antiarrhythmic: Bepridil |
↑Bepridil |
Use with caution. Increased bepridil exposure may be associated with life-threatening reactions such as cardiac arrhythmias. |
|
Anticoagulant: Warfarin |
Concentrations of warfarin may be affected. It is recommended that INR (international normalized ratio) be monitored. |
|
|
Anticonvulsants: Carbamazepine, phenobarbital, phenytoin |
↓Amprenavir |
Use with caution. LEXIVA may be less effective due to decreased amprenavir plasma concentrations in patients taking these agents concomitantly. |
|
Antidepressant: Trazodone |
↑Trazodone |
Concomitant use of trazodone and LEXIVA with or without ritonavir may increase plasma concentrations of trazodone. Adverse events of nausea, dizziness, hypotension, and syncope have been observed following coadministration of trazodone and ritonavir. If trazodone is used with a CYP3A4 inhibitor such as LEXIVA, the combination should be used with caution and a lower dose of trazodone should be considered. |
|
Antifungals: Ketoconazole*, itraconazole |
↑Ketoconazole ↑Itraconazole |
Increase monitoring for adverse events due to ketoconazole or itraconazole. LEXIVA: Dose reduction of ketoconazole or itraconazole may be needed for patients receiving more than 400 mg ketoconazole or itraconazole per day. LEXIVA/ritonavir: High doses of ketoconazole or itraconazole (>200 mg/day) are not recommended. |
|
Antimycobacterial: Rifabutin* |
↑Rifabutin and rifabutin metabolite |
A complete blood count should be performed weekly and as clinically indicated in order to monitor for neutropenia in patients receiving LEXIVA and rifabutin. LEXIVA: A dosage reduction of rifabutin by at least half the recommended dose is required. LEXIVA/ritonavir: Dosage reduction of rifabutin by at least 75% of the usual dose of 300 mg/day is recommended (a maximum dose of 150 mg every other day or 3 times per week). |
|
Benzodiazepines: Alprazolam, clorazepate, diazepam, flurazepam |
↑Benzodiazepines |
Clinical significance is unknown; however, a decrease in benzodiazepine dose may be needed. |
|
Calcium channel blockers: Diltiazem, felodipine, nifedipine, nicardipine, nimodipine, verapamil, amlodipine, nisoldipine, isradipine |
↑Calcium channel blockers |
Caution is warranted and clinical monitoring of patients is recommended. |
|
Corticosteroid: Dexamethasone |
↓Amprenavir |
Use with caution. LEXIVA may be less effective due to decreased amprenavir plasma concentrations in patients taking these agents concomitantly. |
|
Histamine H2-receptor antagonists: Cimetidine, famotidine, nizatidine, ranitidine* |
LEXIVA: ↓Amprenavir LEXIVA/ritonavir: Interaction not evaluated |
Use with caution. LEXIVA may be less effective due to decreased amprenavir plasma concentrations in patients taking these agents concomitantly. |
|
HMG-CoA reductase inhibitor: Atorvastatin* |
↑Atorvastatin |
Use ≤20 mg/day of atorvastatin with careful monitoring, or consider other HMG-CoA reductase inhibitors such as fluvastatin, pravastatin, or rosuvastatin in combination with LEXIVA. |
|
Immunosuppressants: Cyclosporine, tacrolimus, rapamycin |
↑Immunosup-pressants |
Therapeutic concentration monitoring is recommended for immunosuppressant agents when coadministered with LEXIVA. |
|
Inhaled/nasal steroid: Fluticasone |
LEXIVA: ↑Fluticasone LEXIVA/ritonavir: ↑Fluticasone |
Concomitant use of fluticasone propionate and LEXIVA (without ritonavir) may increase plasma concentrations of fluticasone propionate. Use with caution. Consider alternatives to fluticasone propionate, particularly for long-term use. Concomitant use of fluticasone propionate and LEXIVA/ritonavir may increase plasma concentrations of fluticasone propionate, resulting in significantly reduced serum cortisol concentrations. Coadministration of fluticasone propionate and LEXIVA/ritonavir is not recommended unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects (see WARNINGS). |
|
Narcotic analgesic: Methadone |
↓Methadone |
Dosage of methadone may need to be increased when coadministered with LEXIVA. |
|
PDE5 inhibitors: Sildenafil, vardenafil |
↑Sildenafil ↑Vardenafil |
Use sildenafil with caution at reduced doses of 25 mg every 48 hours with increased monitoring for adverse events. LEXIVA: Use vardenafil with caution at reduced doses of no more than 2.5 mg every 24 hours with increased monitoring for adverse events. LEXIVA/ritonavir: Use vardenafil with caution at reduced doses of no more than 2.5 mg every 72 hours with increased monitoring for adverse events. |
|
Proton pump inhibitors: Esomeprazole*, lansoprazole, omeprazole, pantoprazole, rabeprazole |
LEXIVA: ↔Amprenavir ↑Esomeprazole LEXIVA/ritonavir: ↔Amprenavir ↔Esomeprazole |
Proton pump inhibitors can be administered at the same time as a dose of LEXIVA with no change in plasma amprenavir concentrations. |
|
Tricyclic antidepressants: Amitriptyline, imipramine |
↑Tricyclics |
Therapeutic concentration monitoring is recommended for tricyclic antidepressants when coadministered with LEXIVA. |
*See CLINICAL PHARMACOLOGY Tables 3, 4, 5, or 6 for magnitude of interaction.
Carcinogenesis and Mutagenesis
Carcinogenicity studies of fosamprenavir in rats and mice are in progress; however, results are available from carcinogenicity studies with amprenavir. Amprenavir was evaluated for carcinogenic potential by oral gavage administration to mice and rats for up to 104 weeks. Results showed an increase in the incidence of benign hepatocellular adenomas and an increase in the combined incidence of hepatocellular adenomas plus carcinoma in males of both species at the highest doses tested. Female mice and rats were not affected. These observations were made at systemic exposures equivalent to approximately 2 times (mice) and 4 times (rats) the human exposure (based on AUC0-24 hr measurement) at the recommended dose of 1,200 mg twice daily. Administration of amprenavir did not cause a statistically significant increase in the incidence of any other benign or malignant neoplasm in mice or rats. It is not known how predictive the results of rodent carcinogenicity studies may be for humans.
Fosamprenavir and amprenavir were not mutagenic or genotoxic in a battery of in vitro and in vivo assays. These assays included bacterial reverse mutation (Ames), mouse lymphoma, rat micronucleus, and chromosome aberrations in human lymphocytes.
Impairment of Fertility
The effects of fosamprenavir on fertility and general reproductive performance were investigated in male (treated for 4 weeks before mating) and female rats (treated for 2 weeks before mating through postpartum day 6). Systemic exposures (AUC0-24 hr) to amprenavir in these studies were 3 (males) to 4 (females) times higher than exposures in humans following administration of the maximum recommended human dose (MRHD) of fosamprenavir alone or similar to those seen in humans following administration of fosamprenavir in combination with ritonavir. Fosamprenavir did not impair mating or fertility of male or female rats and did not affect the development and maturation of sperm from treated rats.
Pregnancy and Reproduction
Pregnancy Category C. Embryo/fetal development studies were conducted in rats (dosed from day 6 to day 17 of gestation) and rabbits (dosed from day 7 to day 20 of gestation). Administration of fosamprenavir to pregnant rats and rabbits produced no major effects on embryo-fetal development; however, the incidence of abortion was increased in rabbits that were administered fosamprenavir. Systemic exposures (AUC0-24 hr) to amprenavir at these dosages were 0.8 (rabbits) to 2 (rats) times the exposures in humans following administration of the MRHD of fosamprenavir alone or 0.3 (rabbits) to 0.7 (rats) times the exposures in humans following administration of the MRHD of fosamprenavir in combination with ritonavir. In contrast, administration of amprenavir was associated with abortions and an increased incidence of minor skeletal variations resulting from deficient ossification of the femur, humerus, and trochlea, in pregnant rabbits at the tested dose; approximately one twentieth the exposure seen at the recommended human dose.
The mating and fertility of the F1 generation born to female rats given fosamprenavir was not different from control animals; however, fosamprenavir did cause a reduction in both pup survival and body weights. Surviving F1 female rats showed an increased time to successful mating, an increased length of gestation, a reduced number of uterine implantation sites per litter, and reduced gestational body weights compared to control animals. Systemic exposure (AUC0-24 hr) to amprenavir in the F0 pregnant rats was approximately 2 times higher than exposures in humans following administration of the MRHD of fosamprenavir alone or approximately the same as those seen in humans following administration of the MRHD of fosamprenavir in combination with ritonavir.
There are no adequate and well-controlled studies in pregnant women. LEXIVA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Antiretroviral Pregnancy Registry
To monitor maternal-fetal outcomes of pregnant women exposed to LEXIVA, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling 1-800-258-4263.
Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. Although it is not known if amprenavir is excreted in human milk, amprenavir is secreted into the milk of lactating rats. Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving LEXIVA.
Pediatric Use
The safety and efficacy of LEXIVA Tablets have not been established in pediatric patients.
Geriatric Use
Clinical studies of LEXIVA did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger adults. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
LEXIVA was studied in 700 patients in Phase III controlled clinical studies. The most common treatment-emergent adverse events in clinical studies of LEXIVA were diarrhea, nausea, vomiting, headache, and rash and were generally mild to moderate in severity. Treatment discontinuation due to adverse events occurred in 6.4% of patients receiving LEXIVA and in 5.9% of patients receiving comparator treatments.
Severe or life-threatening skin reactions, including 1 case of Stevens-Johnson syndrome among 700 patients treated with LEXIVA, were reported in <1% of patients treated with LEXIVA in the clinical studies. Treatment with LEXIVA should be discontinued for severe or life-threatening rashes and for moderate rashes accompanied by systemic symptoms.
Skin rash (without regard to causality) occurred in approximately 19% of patients treated with LEXIVA in the pivotal efficacy studies. Rashes were usually maculopapular and of mild or moderate intensity, some with pruritus. Rash had a median onset of 11 days after initiation of LEXIVA and had a median duration of 13 days. Skin rash led to discontinuation of LEXIVA in <1% of patients. In some patients with mild or moderate rash, dosing with LEXIVA was often continued without interruption; if interrupted, reintroduction of LEXIVA generally did not result in rash recurrence.
Selected adverse events reported during the clinical efficacy studies of LEXIVA are shown in Tables 14 and 15. Each table presents drug-related adverse events of moderate or severe intensity and adverse events of all grades regardless of causality in patients treated with combination therapy for up to 48 weeks.
|
APV30001* |
APV30002* |
|||||||
|
LEXIVA 1,400 mg b.i.d. (n = 166) |
Nelfinavir 1,250 mg b.i.d. (n = 83) |
LEXIVA 1,400 mg q.d./Ritonavir 200 mg q.d. (n = 322) |
Nelfinavir 1,250 mg b.i.d. (n = 327) |
|||||
|
Adverse Event |
Moderate/ Severe Drug-Related |
All Grades† |
Moderate/ Severe Drug-Related |
All Grades† |
Moderate/ Severe Drug-Related |
All Grades† |
Moderate/ Severe Drug-Related |
All Grades† |
|
Gastrointestinal |
||||||||
|
Diarrhea |
5% |
34% |
18% |
63% |
10% |
52% |
18% |
72% |
|
Nausea |
7% |
39% |
4% |
24% |
7% |
37% |
5% |
27% |
|
Vomiting |
2% |
16% |
4% |
17% |
6% |
20% |
4% |
13% |
|
Abdominal pain |
1% |
5% |
0% |
8% |
2% |
11% |
2% |
11% |
|
Skin |
||||||||
|
Pruritus |
0% |
7% |
0% |
11% |
<1% |
7% |
1% |
9% |
|
Rash |
8% |
35% |
2% |
19% |
3% |
17% |
2% |
21% |
|
General disorders |
||||||||
|
Fatigue |
2% |
10% |
1% |
7% |
4% |
18% |
2% |
13% |
|
Nervous system |
||||||||
|
Depressive/ mood disorders |
1% |
8% |
0% |
8% |
<1% |
8% |
0% |
6% |
|
Headache |
2% |
19% |
4% |
20% |
3% |
21% |
3% |
27% |
|
Paresthesia, oral |
0% |
2% |
0% |
0% |
<1% |
10% |
0% |
<1% |
*All patients also received abacavir and lamivudine twice daily.
†Includes adverse events of all grades regardless of causality reported in >5% of patients.
|
LEXIVA 700 mg b.i.d./ Ritonavir 100 mg b.i.d.* (n = 106) |
Lopinavir 400 mg b.i.d./ Ritonavir 100 mg b.i.d.* (n = 103) |
|||
|
Adverse Event |
Moderate/Severe Drug-Related |
All Grades† |
Moderate/Severe Drug-Related |
All Grades† |
|
Gastrointestinal |
||||
|
Diarrhea |
13% |
38% |
11% |
47% |
|
Nausea |
3% |
20% |
9% |
31% |
|
Vomiting |
3% |
10% |
5% |
17% |
|
Abdominal pain |
<1% |
11% |
2% |
9% |
|
Skin |
||||
|
Pruritus |
<1% |
8% |
0% |
3% |
|
Rash |
3% |
9% |
0% |
22% |
|
General disorders |
||||
|
Fatigue |
<1% |
9% |
<1% |
14% |
|
Nervous system |
||||
|
Depressive/mood disorders |
<1% |
11% |
<1% |
10% |
|
Headache |
4% |
27% |
2% |
20% |
|
Paresthesia, oral |
0% |
<1% |
0% |
0% |
*All patients also received 2 reverse transcriptase inhibitors.
†Includes adverse events of all grades regardless of causality in >5% of patients.
The percentages of patients with Grade 3 or 4 laboratory abnormalities in the clinical efficacy studies of LEXIVA are presented in Tables 16 and 17.
|
APV30001* |
APV30002* |
|||
|
Laboratory Abnormality |
LEXIVA 1,400 mg b.i.d. (n = 166) |
Nelfinavir 1,250 mg b.i.d. (n = 83) |
LEXIVA 1,400 mg q.d./ Ritonavir 200 mg q.d. (n = 322) |
Nelfinavir 1,250 mg b.i.d. (n = 327) |
|
ALT (>5 x ULN) |
6% |
5% |
8% |
8% |
|
AST (>5 x ULN) |
6% |
6% |
6% |
7% |
|
Serum lipase (>2 x ULN) |
8% |
4% |
6% |
4% |
|
Hypertriglyceridemia† (>750 mg/dL) |
0% |
1% |
6% |
2% |
|
Neutropenia (<750 cells/mm3) |
3% |
6% |
3% |
4% |
*All patients also received abacavir and lamivudine twice daily.
†Fasting specimens.
ULN = Upper limit of normal.
The incidence of Grade 3 or 4 hyperglycemia in antiretroviral-naive patients who received LEXIVA in the pivotal studies was <1%.
|
Laboratory Abnormality |
LEXIVA 700 mg b.i.d./ Ritonavir 100 mg b.i.d.* (n = 104) |
Lopinavir 400 mg b.i.d./ Ritonavir 100 mg b.i.d.* (n = 103) |
|
Hypertriglyceridemia† (>750 mg/dL) |
11%‡ |
6%‡ |
|
Serum lipase (>2 x ULN) |
5% |
12% |
|
ALT (>5 x ULN) |
4% |
4% |
|
AST (>5 x ULN) |
4% |
2% |
|
Hyperglycemia (>251 mg/dL) |
2%‡ |
2%‡ |
*All patients also received 2 reverse transcriptase inhibitors.
†Fasting specimens.
‡n = 100 for LEXIVA/ritonavir, n = 98 for lopinavir/ritonavir.
ULN = Upper limit of normal.
OVERDOSAGE
In a healthy volunteer repeat-dose pharmacokinetic study evaluating high-dose combinations of LEXIVA plus ritonavir, an increased frequency of Grade 2/3 ALT elevations (>2.5 x ULN) was observed with LEXIVA 1,400 mg twice daily plus ritonavir 200 mg twice daily (4 of 25 subjects). Concurrent Grade 1/2 elevations in AST (>1.25 x ULN) were noted in 3 of these 4 subjects. These transaminase elevations resolved following discontinuation of dosing.
There is no known antidote for LEXIVA. It is not known whether amprenavir can be removed by peritoneal dialysis or hemodialysis. If overdosage occurs, the patient should be monitored for evidence of toxicity and standard supportive treatment applied as necessary.
DOSAGE AND ADMINISTRATION
LEXIVA Tablets may be taken with or without food.
The recommended oral dose of LEXIVA, alone or in combination with ritonavir, is as follows:
Therapy-Naive Patients
- LEXIVA 1,400 mg twice daily (without ritonavir)
- LEXIVA 1,400 mg once daily plus ritonavir 200 mg once daily
- LEXIVA 700 mg twice daily plus ritonavir 100 mg twice daily
The twice-daily plus ritonavir dose is supported by pharmacokinetic and safety data (see CLINICAL PHARMACOLOGY and ADVERSE REACTIONS).
Protease Inhibitor-Experienced Patients
- LEXIVA 700 mg twice daily plus ritonavir 100 mg twice daily
Once-daily administration of LEXIVA plus ritonavir is not recommended in protease inhibitor-experienced patients (see Description of Clinical Studies).
Adjustment of Ritonavir Dose When LEXIVA Plus Ritonavir are Administered With Efavirenz
An additional 100 mg/day (300 mg total) of ritonavir is recommended when efavirenz is administered with LEXIVA plus ritonavir once daily (see Table 13. Established and Other Potentially Significant Drug Interactions: Alteration in Dose or Regimen May Be Recommended Based on Drug Interaction Studies or Predicted Interaction).
Prescribers should consult the full prescribing information for NORVIR (ritonavir) when using this agent.
High-Dose Combinations of LEXIVA Plus Ritonavir
Higher-than-approved dose combinations of LEXIVA plus ritonavir are not recommended for use (see PRECAUTIONS and OVERDOSAGE).
Patients With Hepatic Impairment
LEXIVA Tablets should be used with caution, at a reduced dosage of 700 mg twice daily in patients with mild or moderate hepatic impairment (Child-Pugh score ranging from 5 to 8) receiving LEXIVA without concurrent ritonavir (see CLINICAL PHARMACOLOGY: Hepatic Insufficiency). LEXIVA should not be used in patients with severe hepatic impairment (Child-Pugh score ranging from 9 to 12) because the dose cannot be reduced below 700 mg. There are no data on the use of LEXIVA in combination with ritonavir in patients with any degree of hepatic impairment.
HOW SUPPLIED
LEXIVA Tablets, 700 mg, are pink, film-coated, capsule-shaped, biconvex tablets, with “GX LL7” debossed on one face.
Bottles of 60 with child-resistant closures (NDC 0173-0721-00).
Store at controlled room temperature of 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) (see USP Controlled Room Temperature). Keep container tightly closed.
PHARMACIST-DETACH HERE AND GIVE INSTRUCTIONS TO PATIENT
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ __ _ _ _ _ _ _ _ _
PATIENT INFORMATION
LEXIVA®
(lex-EE-vah)
(fosamprenavir calcium) Tablets
Read the Patient Information that comes with LEXIVA before you start taking it and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment. It is important to remain under a healthcare provider's care while taking LEXIVA. Do not change or stop treatment without first talking with your healthcare provider. Talk to your healthcare provider or pharmacist if you have any questions about LEXIVA.
What is the most important information I should know about LEXIVA?
LEXIVA cancause dangerous and life-threatening interactions if taken with certain other medicines. Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
- Some medicines cannot be taken at all with LEXIVA.
- Some medicines will require dose changes if taken with LEXIVA.
- Some medicines will require close monitoring if you take them with LEXIVA.
Know all the medicines you take, including prescription and nonprescription medicines, vitamins and herbal supplements. Keep a list of the medicines you take. Show this list to all your healthcare providers and pharmacists anytime you get a new medicine or refill. Your healthcare providers and pharmacists must know all the medicines you take. They will tell you if you can take other medicines with LEXIVA. Do not start any new medicines while you are taking LEXIVA without talking with your healthcare provider or pharmacist. You can ask your healthcare provider or pharmacist for a list of medicines that can interact with LEXIVA.
What is LEXIVA?
LEXIVA is a medicine you take by mouth to treat HIV infection. HIV is the virus that causes AIDS (acquired immune deficiency syndrome.) LEXIVA belongs to a class of anti-HIV medicines called protease inhibitors. LEXIVA is always used with other anti-HIV medicines. When used in combination therapy, LEXIVA may help lower the amount of HIV found in your blood, raise CD4+ (T) cell counts, and keep your immune system as healthy as possible, so it can help fight infection. However, LEXIVA does not work in all patients with HIV.
LEXIVA does not:
- cure HIV infection or AIDS. We do not know if LEXIVA will help you live longer or have fewer of the medical problems (opportunistic infections) that people get with HIV or AIDS. Opportunistic infections are infections that develop because the immune system is weak. Some of these conditions are pneumonia, herpes virus infections, and Mycobacterium aviumcomplex (MAC) infections. It is very important that you see your healthcare provider regularly while you are taking LEXIVA. The long-term effects of LEXIVA are not known.
- lower the risk of passing HIV to other people through sexual contact, sharing needles, or being exposed to your blood. For your health and the health of others, it is important to always practice safer sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood. Never use or share dirty needles.
LEXIVA has not been fully studied in children under the age of 18 or in adults over the age of 65.
Who should not take LEXIVA?
Do not take LEXIVA Tablets if you:
- are taking certain other medicines. Read the section “What
is the most important information I should know about LEXIVA?”Do not take the following medicines* with LEXIVA.
You could develop serious or life-threatening problems.
- HALCION® (triazolam; used for insomnia)
- Ergot medicines: dihydroergotamine, ergonovine, ergotamine, and methylergonovine such as CAFERGOT®, MIGRANAL®, D.H.E. 45®, ergotrate maleate, METHERGINE®, and others (used for migraine headaches)
- PROPULSID® (cisapride), used for certain stomach problems
- VERSED® (midazolam), used for sedation
- ORAP® (pimozide), used for Tourette’s disorder
Do not take the following medicines if you are taking LEXIVA and NORVIR® (ritonavir) together. You could develop serious or life-threatening problems.
- TAMBOCOR® (flecainide), used for certain abnormal heart
rhythms
- RYTHMOL® (propafenone), used for certain abnormal heart rhythms
- are allergic to LEXIVA or any of its ingredients. The active ingredient is fosamprenavir calcium. See the end of this leaflet for a list of all the ingredients in LEXIVA.
- are allergic to AGENERASE® (amprenavir).
You should not take AGENERASE (amprenavir) and LEXIVA at the same time.
Before taking LEXIVA, tell your healthcare provider about all your medical conditions including if you:
- are pregnant or planning to become pregnant. It is not known if LEXIVA can harm your unborn baby. You and your healthcare provider will need to decide if LEXIVA is right for you. If you use LEXIVA while you are pregnant, talk to your healthcare provider about how you can be on the Antiretroviral Pregnancy Registry.
- are breastfeeding. You should not breastfeed if you are HIV-positive because of the chance of passing the HIV virus to your baby through your milk. Also, it is not known if LEXIVA can pass into your breast milk and if it can harm your baby. If you are a woman who has or will have a baby, talk with your healthcare provider about the best way to feed your baby.
- have liver problems. You may be given a lower dose of LEXIVA or LEXIVA may not be right for you.
- have kidney problems
- have diabetes. You may need dose changes in your insulin or other diabetes medicines.
- have hemophilia
- are allergic to sulfa medicines
Before taking LEXIVA, tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. LEXIVA can cause dangerous and life-threatening interactions if taken with certain other medicines. You may need dose changes in some of your medicines or closer monitoring with some medicines if you also take LEXIVA (see “What is the most important information I should know about LEXIVA.”). Know all the medicines that you take and keep a list of them with you to show healthcare providers and pharmacists.
Women who use birth control pills should choose a different kind of contraception. The use of LEXIVA with NORVIR (ritonavir) in combination with birth control pills may be harmful to your liver. The use of LEXIVA with or without NORVIR may decrease the effectiveness of birth control pills. Talk to your healthcare provider about choosing an effective contraceptive.
How should I take LEXIVA?
- Take LEXIVA Tablets exactly as your healthcare provider prescribed.
- Do not take more or less than your prescribed dose of LEXIVA Tablets at any one time. Do not change your dose or stop taking LEXIVA without talking with your healthcare provider.
- You can take LEXIVA Tablets with or without food.
- When your supply of LEXIVA or other anti-HIV medicine starts to run low, get more from your healthcare provider or pharmacy. The amount of HIV virus in your blood may increase if one or more of the medicines are stopped, even for a short time.
- Stay under the care of a healthcare provider while using LEXIVA.
- It is important that you do not miss any doses. If you miss a dose of LEXIVA by more than 4 hours, wait and take the next dose at the regular time. However, if you miss a dose by fewer than 4 hours, take your missed dose right away. Then take your next dose at the regular time.
- If you take too much LEXIVA, call your healthcare provider or poison control center right away.
What should I avoid while taking LEXIVA?
- Do not use certain medicines while you are taking LEXIVA. See “What is the most important information I should know about LEXIVA" and "Who should not take LEXIVA?”
- Do not breastfeed. See “Before taking LEXIVA, tell your healthcare provider”. Talk with your healthcare provider about the best way to feed your baby.
- Avoid doing things that can spread HIV infection since LEXIVA doesn't stop you from passing the HIV infection to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes or razor blades.
- Do not have any kind of sex without protection. Always practice safer sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
What are the possible side effects of LEXIVA?
LEXIVA may cause the following side effects:
- skin rash. Skin rashes, some with itching, have happened in patients taking LEXIVA.Tell your healthcare provider if you get a rash after starting LEXIVA.
- diabetes and high blood sugar (hyperglycemia).Some patients had diabetes before taking LEXIVA while others did not. Some patients may need changes in their diabetes medicine. Others may need a new diabetes medicine.
- increased bleeding problems in some patients with hemophilia.
- worse liver disease. Patients with liver problems, including hepatitis B or C, are more likely to get worse liver disease when they take anti-HIV medicines like LEXIVA.
- changes in blood tests. Some people have changes in blood tests while taking LEXIVA. These include increases seen in liver function tests and blood fat levels, and decreases in white blood cells. Your healthcare provider may do regular blood tests to see if LEXIVA is affecting your body.
- changes in body fat. These changes have happened in patients taking antiretroviral medicines like LEXIVA. The changes may include an increased amount of fat in the upper back and neck ("buffalo hump"), breast, and around the trunk. Loss of fat from the legs, arms, and face may also happen. The cause and long-term health effects of these conditions are not known at this time.
Common side effects of LEXIVA are nausea, vomiting, and diarrhea. Tell your healthcare provider about any side effects that bother you or that won't go away.
This list of side effects of LEXIVA is not complete. For more information, ask your healthcare provider or pharmacist.
How should I store LEXIVA Tablets?
- Store LEXIVA Tablets at room temperature between 59° and 86°F (15° to 30°C). Keep the container tightly closed.
- Keep LEXIVA and all medicines out of the reach of children.
- Do not keep medicine that is out of date or that you no longer need. Be sure that if you throw any medicine away, it is out of the reach of children.
General information about LEXIVA
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use LEXIVA for a condition for which it was not prescribed. Do not give LEXIVA to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about LEXIVA. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about LEXIVA that is written for health professionals. For more information you can call toll-free 888-825-5249 or visit www.LEXIVA.com.
What are the ingredients in LEXIVA?
Active Ingredient:fosamprenavir calcium.
Inactive Ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and povidone K30. The tablet film-coating contains the inactive ingredients hypromellose, iron oxide red, titanium dioxide, and triacetin.
LEXIVA Tablets, 700 mg, are pink in color and are capsule-shaped, with the letters “GX LL7” printed on one side of the tablet.

Rx only
LEXIVA is a registered trademark of GlaxoSmithKline.
* The brands listed are trademarks of their respective owners and are not trademarks of GlaxoSmithKline. The makers of these brands are not affiliated with and do not endorse GlaxoSmithKline or its products.
GlaxoSmithKline Vertex Pharmaceuticals Incorporated
Research Triangle Park, NC 27709 Cambridge, MA 02139
©2006, GlaxoSmithKline. All rights reserved.
November 2006 RL-2343
| Lexiva (fosamprenavir calcium) | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
Revised: 03/2007GlaxoSmithKline