KOMBIGLYZE XR
-
saxagliptin hydrochloride and
metformin hydrochloride tablet, film coated, extended release
E.R. Squibb & Sons, L.L.C.
----------
|
||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
WARNING: LACTIC ACIDOSIS
Lactic acidosis is a rare, but serious, complication that can occur due to metformin accumulation. The risk increases with conditions such as sepsis, dehydration, excess alcohol intake, hepatic impairment, renal impairment, and acute congestive heart failure.
The onset of lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress.
Laboratory abnormalities include low pH, increased anion gap, and elevated blood lactate.
If acidosis is suspected, KOMBIGLYZE XR should be discontinued and the patient hospitalized immediately. [See Warnings and Precautions (5.1).]
1 INDICATIONS AND USAGE
KOMBIGLYZE XR is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus when treatment with both saxagliptin and metformin is appropriate. [See Clinical Studies (14).]
1.1 Important Limitations of Use
KOMBIGLYZE XR should not be used for the treatment of type 1 diabetes mellitus or diabetic ketoacidosis.
KOMBIGLYZE XR has not been studied in combination with insulin.
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
The dosage of KOMBIGLYZE XR should be individualized on the basis of the patient’s current regimen, effectiveness, and tolerability. KOMBIGLYZE XR should generally be administered once daily with the evening meal, with gradual dose titration to reduce the gastrointestinal side effects associated with metformin. The following dosage forms are available:
- KOMBIGLYZE XR (saxagliptin and metformin HCl extended-release) tablets 5 mg/500 mg
- KOMBIGLYZE XR (saxagliptin and metformin HCl extended-release) tablets 5 mg/1000 mg
- KOMBIGLYZE XR (saxagliptin and metformin HCl extended-release) tablets 2.5 mg/1000 mg
The recommended starting dose of KOMBIGLYZE XR in patients who need 5 mg of saxagliptin and who are not currently treated with metformin is 5 mg saxagliptin/500 mg metformin extended-release once daily with gradual dose escalation to reduce the gastrointestinal side effects due to metformin.
In patients treated with metformin, the dose of KOMBIGLYZE XR should provide metformin at the dose already being taken, or the nearest therapeutically appropriate dose. Following a switch from metformin immediate-release to metformin extended-release, glycemic control should be closely monitored and dosage adjustments made accordingly.
Patients who need 2.5 mg saxagliptin in combination with metformin extended-release may be treated with KOMBIGLYZE XR 2.5 mg/1000 mg. Patients who need 2.5 mg saxagliptin who are either metformin naive or who require a dose of metformin higher than 1000 mg should use the individual components.
The maximum daily recommended dose is 5 mg for saxagliptin and 2000 mg for metformin extended-release.
No studies have been performed specifically examining the safety and efficacy of KOMBIGLYZE XR in patients previously treated with other antihyperglycemic medications and switched to KOMBIGLYZE XR. Any change in therapy of type 2 diabetes should be undertaken with care and appropriate monitoring as changes in glycemic control can occur.
Inform patients that KOMBIGLYZE XR tablets must be swallowed whole and never crushed, cut, or chewed. Occasionally, the inactive ingredients of KOMBIGLYZE XR will be eliminated in the feces as a soft, hydrated mass that may resemble the original tablet.
2.2 Strong CYP3A4/5 Inhibitors
The maximum recommended dose of saxagliptin is 2.5 mg once daily when coadministered with strong cytochrome P450 3A4/5 (CYP3A4/5) inhibitors (e.g., ketoconazole, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, and telithromycin). For these patients, limit the KOMBIGLYZE XR dose to 2.5 mg/1000 mg once daily. [See Dosage and Administration (2.1), Drug Interactions (7.1), and Clinical Pharmacology (12.3).]
3 DOSAGE FORMS AND STRENGTHS
- KOMBIGLYZE XR (saxagliptin and metformin HCl extended-release) 5 mg/500 mg tablets are light brown to brown, biconvex, capsule-shaped, film-coated tablets with “5/500” printed on one side and “4221” printed on the reverse side, in blue ink.
- KOMBIGLYZE XR (saxagliptin and metformin HCl extended-release) 5 mg/1000 mg tablets are pink, biconvex, capsule-shaped, film-coated tablets with “5/1000” printed on one side and “4223” printed on the reverse side, in blue ink.
- KOMBIGLYZE XR (saxagliptin and metformin HCl extended-release) 2.5 mg/1000 mg tablets are pale yellow to light yellow, biconvex, capsule-shaped, film-coated tablets with “2.5/1000” printed on one side and “4222” printed on the reverse side, in blue ink.
4 CONTRAINDICATIONS
KOMBIGLYZE XR is contraindicated in patients with:
- Renal impairment (e.g., serum creatinine levels ≥1.5 mg/dL for men, ≥1.4 mg/dL for women, or abnormal creatinine clearance) which may also result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, and septicemia.
- Hypersensitivity to metformin hydrochloride.
- Acute or chronic metabolic acidosis, including diabetic ketoacidosis. Diabetic ketoacidosis should be treated with insulin.
KOMBIGLYZE XR should be temporarily discontinued in patients undergoing radiologic studies involving intravascular administration of iodinated contrast materials because use of such products may result in acute alteration of renal function [see Warnings and Precautions (5.10)].
5 WARNINGS AND PRECAUTIONS
5.1 Lactic Acidosis
Lactic acidosis is a rare, but serious, metabolic complication that can occur due to metformin accumulation during treatment with KOMBIGLYZE XR; when it occurs, it is fatal in approximately 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by elevated blood lactate levels (>5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels >5 µg/mL are generally found.
The reported incidence of lactic acidosis in patients receiving metformin hydrochloride is very low (approximately 0.03 cases/1000 patient-years, with approximately 0.015 fatal cases/1000 patient-years). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, in particular those with unstable or acute congestive heart failure who are at risk of hypoperfusion and hypoxemia, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal dysfunction and the patient’s age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin and by use of the minimum effective dose of metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Metformin treatment should not be initiated in patients ≥80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced, as these patients are more susceptible to developing lactic acidosis. In addition, metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, metformin should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Patients should be cautioned against excessive alcohol intake when taking metformin since alcohol potentiates the effects of metformin hydrochloride on lactate metabolism. In addition, metformin should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure [see Warnings and Precautions (5.2, 5.5, 5.6, 5.10)].
The onset of lactic acidosis often is subtle and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. There may be associated hypothermia, hypotension, and resistant bradyarrhythmias with more marked acidosis. The patient and the patient’s physician must be aware of the possible importance of such symptoms and the patient should be instructed to notify the physician immediately if they occur [see Warnings and Precautions (5.11)]. Metformin should be withdrawn until the situation is clarified. Serum electrolytes, ketones, blood glucose, and if indicated, blood pH, lactate levels, and even blood metformin levels may be useful. Once a patient is stabilized on any dose level of metformin, gastrointestinal symptoms, which are common during initiation of therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
Levels of fasting venous plasma lactate above the upper limit of normal, but less than 5 mmol/L, in patients taking metformin do not necessarily indicate impending lactic acidosis and may be explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous physical activity, or technical problems in sample handling. [See Warnings and Precautions (5.7).]
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia).
Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking metformin, the drug should be discontinued immediately and general supportive measures promptly instituted. Because metformin hydrochloride is dialyzable (with a clearance of up to 170 mL/min under good hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis and remove the accumulated metformin. Such management often results in prompt reversal of symptoms and recovery [see Contraindications (4) and Warnings and Precautions (5.5, 5.6, 5.9, 5.10, 5.11)].
5.2 Assessment of Renal Function
Metformin is substantially excreted by the kidney, and the risk of metformin accumulation and lactic acidosis increases with the degree of impairment of renal function. Therefore, KOMBIGLYZE XR is contraindicated in patients with renal impairment [see Contraindications (4)].
Before initiation of KOMBIGLYZE XR, and at least annually thereafter, renal function should be assessed and verified as normal. In patients in whom development of renal impairment is anticipated (e.g., elderly), renal function should be assessed more frequently and KOMBIGLYZE XR discontinued if evidence of renal impairment is present.
5.3 Impaired Hepatic Function
Metformin use in patients with impaired hepatic function has been associated with some cases of lactic acidosis. Therefore, KOMBIGLYZE XR is not recommended in patients with hepatic impairment.
5.4 Vitamin B12 Concentrations
In controlled clinical trials of metformin of 29-week duration, a decrease to subnormal levels of previously normal serum vitamin B12 levels, without clinical manifestations, was observed in approximately 7% of patients. Such decrease, possibly due to interference with B12 absorption from the B12-intrinsic factor complex, is, however, very rarely associated with anemia and appears to be rapidly reversible with discontinuation of metformin or vitamin B12 supplementation. Measurement of hematologic parameters on an annual basis is advised in patients on KOMBIGLYZE XR and any apparent abnormalities should be appropriately investigated and managed [see Adverse Reactions (6.1)].
Certain individuals (those with inadequate vitamin B12 or calcium intake or absorption) appear to be predisposed to developing subnormal vitamin B12 levels. In these patients, routine serum vitamin B12 measurements at 2- to 3-year intervals may be useful.
5.5 Alcohol Intake
Alcohol potentiates the effect of metformin on lactate metabolism. Patients should be warned against excessive alcohol intake while receiving KOMBIGLYZE XR.
5.6 Surgical Procedures
Use of KOMBIGLYZE XR should be temporarily suspended for any surgical procedure (except minor procedures not associated with restricted intake of food and fluids) and should not be restarted until the patient’s oral intake has resumed and renal function has been evaluated as normal.
5.7 Change in Clinical Status of Patients with Previously Controlled Type 2 Diabetes
A patient with type 2 diabetes previously well controlled on KOMBIGLYZE XR who develops laboratory abnormalities or clinical illness (especially vague and poorly defined illness) should be evaluated promptly for evidence of ketoacidosis or lactic acidosis. Evaluation should include serum electrolytes and ketones, blood glucose and, if indicated, blood pH, lactate, pyruvate, and metformin levels. If acidosis of either form occurs, KOMBIGLYZE XR must be stopped immediately and other appropriate corrective measures initiated.
5.8 Use with Medications Known to Cause Hypoglycemia
Saxagliptin
Insulin secretagogues, such as sulfonylureas, cause hypoglycemia. Therefore, when used in combination with saxagliptin, a lower dose of the insulin secretagogue may be required to reduce the risk of hypoglycemia. [See Adverse Reactions (6.1).]
Metformin hydrochloride
Hypoglycemia does not occur in patients receiving metformin alone under usual circumstances of use, but could occur when caloric intake is deficient, when strenuous exercise is not compensated by caloric supplementation, or during concomitant use with other glucose-lowering agents (such as sulfonylureas and insulin) or ethanol. Elderly, debilitated, or malnourished patients and those with adrenal or pituitary insufficiency or alcohol intoxication are particularly susceptible to hypoglycemic effects. Hypoglycemia may be difficult to recognize in the elderly and in people who are taking beta-adrenergic blocking drugs.
5.9 Concomitant Medications Affecting Renal Function or Metformin Disposition
Concomitant medication(s) that may affect renal function or result in significant hemodynamic change or may interfere with the disposition of metformin, such as cationic drugs that are eliminated by renal tubular secretion [see Drug Interactions (7.2)], should be used with caution.
5.10 Radiologic Studies with Intravascular Iodinated Contrast Materials
Intravascular contrast studies with iodinated materials can lead to acute alteration of renal function and have been associated with lactic acidosis in patients receiving metformin [see Contraindications (4)]. Therefore, in patients in whom any such study is planned, KOMBIGLYZE XR should be temporarily discontinued at the time of or prior to the procedure, and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been re-evaluated and found to be normal.
5.11 Hypoxic States
Cardiovascular collapse (shock), acute congestive heart failure, acute myocardial infarction, and other conditions characterized by hypoxemia have been associated with lactic acidosis and may also cause prerenal azotemia. When such events occur in patients on KOMBIGLYZE XR therapy, the drug should be promptly discontinued.
5.12 Macrovascular Outcomes
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with KOMBIGLYZE XR or any other antidiabetic drug.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Monotherapy and Add-On Combination Therapy
Metformin hydrochloride
In placebo-controlled monotherapy trials of metformin extended-release, diarrhea and nausea/vomiting were reported in >5% of metformin-treated patients and more commonly than in placebo-treated patients (9.6% versus 2.6% for diarrhea and 6.5% versus 1.5% for nausea/vomiting). Diarrhea led to discontinuation of study medication in 0.6% of the patients treated with metformin extended-release.
Saxagliptin
In two placebo-controlled monotherapy trials of 24-week duration, patients were treated with saxagliptin 2.5 mg daily, saxagliptin 5 mg daily, and placebo. Three 24-week, placebo-controlled, add-on combination therapy trials were also conducted: one with metformin immediate-release, one with a thiazolidinedione (pioglitazone or rosiglitazone), and one with glyburide. In these three trials, patients were randomized to add-on therapy with saxagliptin 2.5 mg daily, saxagliptin 5 mg daily, or placebo. A saxagliptin 10 mg treatment arm was included in one of the monotherapy trials and in the add-on combination trial with metformin immediate-release.
In a prespecified pooled analysis of the 24-week data (regardless of glycemic rescue) from the two monotherapy trials, the add-on to metformin immediate-release trial, the add-on to thiazolidinedione (TZD) trial, and the add-on to glyburide trial, the overall incidence of adverse events in patients treated with saxagliptin 2.5 mg and saxagliptin 5 mg was similar to placebo (72.0% and 72.2% versus 70.6%, respectively). Discontinuation of therapy due to adverse events occurred in 2.2%, 3.3%, and 1.8% of patients receiving saxagliptin 2.5 mg, saxagliptin 5 mg, and placebo, respectively. The most common adverse events (reported in at least 2 patients treated with saxagliptin 2.5 mg or at least 2 patients treated with saxagliptin 5 mg) associated with premature discontinuation of therapy included lymphopenia (0.1% and 0.5% versus 0%, respectively), rash (0.2% and 0.3% versus 0.3%), blood creatinine increased (0.3% and 0% versus 0%), and blood creatine phosphokinase increased (0.1% and 0.2% versus 0%). The adverse reactions in this pooled analysis reported (regardless of investigator assessment of causality) in ≥5% of patients treated with saxagliptin 5 mg, and more commonly than in patients treated with placebo are shown in Table 1.
| Number (%) of Patients | ||
|---|---|---|
| Saxagliptin
5 mg N=882 | Placebo N=799 |
|
| * The 5 placebo-controlled trials include two monotherapy trials and one add-on combination therapy trial with each of the following: metformin, thiazolidinedione, or glyburide. Table shows 24-week data regardless of glycemic rescue. | ||
| Upper respiratory tract infection | 68 (7.7) | 61 (7.6) |
| Urinary tract infection | 60 (6.8) | 49 (6.1) |
| Headache | 57 (6.5) | 47 (5.9) |
In patients treated with saxagliptin 2.5 mg, headache (6.5%) was the only adverse reaction reported at a rate ≥5% and more commonly than in patients treated with placebo.
In this pooled analysis, adverse reactions that were reported in ≥2% of patients treated with saxagliptin 2.5 mg or saxagliptin 5 mg and ≥1% more frequently compared to placebo included: sinusitis (2.9% and 2.6% versus 1.6%, respectively), abdominal pain (2.4% and 1.7% versus 0.5%), gastroenteritis (1.9% and 2.3% versus 0.9%), and vomiting (2.2% and 2.3% versus 1.3%).
The incidence rate of fractures was 1.0 and 0.6 per 100 patient-years, respectively, for saxagliptin (pooled analysis of 2.5 mg, 5 mg, and 10 mg) and placebo. The incidence rate of fracture events in patients who received saxagliptin did not increase over time. Causality has not been established and nonclinical studies have not demonstrated adverse effects of saxagliptin on bone.
An event of thrombocytopenia, consistent with a diagnosis of idiopathic thrombocytopenic purpura, was observed in the clinical program. The relationship of this event to saxagliptin is not known.
Adverse Reactions Associated with Saxagliptin Coadministered with Metformin Immediate-Release in Treatment-Naive Patients with Type 2 Diabetes
Table 2 shows the adverse reactions reported (regardless of investigator assessment of causality) in ≥5% of patients participating in an additional 24-week, active-controlled trial of coadministered saxagliptin and metformin in treatment-naive patients.
| Number (%) of Patients | ||
|---|---|---|
| Saxagliptin
5 mg + Metformin* N=320 | Placebo + Metformin* N=328 |
|
| * Metformin immediate-release was initiated at a starting dose of 500 mg daily and titrated up to a maximum of 2000 mg daily. | ||
| Headache | 24 (7.5) | 17 (5.2) |
| Nasopharyngitis | 22 (6.9) | 13 (4.0) |
In patients treated with the combination of saxagliptin and metformin immediate-release, either as saxagliptin add-on to metformin immediate-release therapy or as coadministration in treatment-naive patients, diarrhea was the only gastrointestinal-related event that occurred with an incidence ≥5% in any treatment group in both studies. In the saxagliptin add-on to metformin immediate-release trial, the incidence of diarrhea was 9.9%, 5.8%, and 11.2% in the saxagliptin 2.5 mg, 5 mg, and placebo groups, respectively. When saxagliptin and metformin immediate-release were coadministered in treatment-naive patients, the incidence of diarrhea was 6.9% in the saxagliptin 5 mg + metformin immediate-release group and 7.3% in the placebo + metformin immediate-release group.
Hypoglycemia
In the saxagliptin clinical trials, adverse reactions of hypoglycemia were based on all reports of hypoglycemia; a concurrent glucose measurement was not required. The incidence of reported hypoglycemia for saxagliptin 2.5 mg and saxagliptin 5 mg versus placebo given as monotherapy was 4.0% and 5.6% versus 4.1%, respectively. In the add-on to metformin immediate-release trial, the incidence of reported hypoglycemia was 7.8% with saxagliptin 2.5 mg, 5.8% with saxagliptin 5 mg, and 5.0% with placebo. When saxagliptin and metformin immediate-release were coadministered in treatment-naive patients, the incidence of reported hypoglycemia was 3.4% in patients given saxagliptin 5 mg + metformin immediate-release and 4.0% in patients given placebo + metformin immediate-release.
Hypersensitivity Reactions
Saxagliptin
Hypersensitivity-related events, such as urticaria and facial edema in the 5-study pooled analysis up to Week 24 were reported in 1.5%, 1.5%, and 0.4% of patients who received saxagliptin 2.5 mg, saxagliptin 5 mg, and placebo, respectively. None of these events in patients who received saxagliptin required hospitalization or were reported as life-threatening by the investigators. One saxagliptin-treated patient in this pooled analysis discontinued due to generalized urticaria and facial edema.
Infections
Saxagliptin
In the unblinded, controlled, clinical trial database for saxagliptin to date, there have been 6 (0.12%) reports of tuberculosis among the 4959 saxagliptin-treated patients (1.1 per 1000 patient-years) compared to no reports of tuberculosis among the 2868 comparator-treated patients. Two of these six cases were confirmed with laboratory testing. The remaining cases had limited information or had presumptive diagnoses of tuberculosis. None of the six cases occurred in the United States or in Western Europe. One case occurred in Canada in a patient originally from Indonesia who had recently visited Indonesia. The duration of treatment with saxagliptin until report of tuberculosis ranged from 144 to 929 days. Post-treatment lymphocyte counts were consistently within the reference range for four cases. One patient had lymphopenia prior to initiation of saxagliptin that remained stable throughout saxagliptin treatment. The final patient had an isolated lymphocyte count below normal approximately four months prior to the report of tuberculosis. There have been no spontaneous reports of tuberculosis associated with saxagliptin use. Causality has not been established and there are too few cases to date to determine whether tuberculosis is related to saxagliptin use.
There has been one case of a potential opportunistic infection in the unblinded, controlled clinical trial database to date in a saxagliptin-treated patient who developed suspected foodborne fatal salmonella sepsis after approximately 600 days of saxagliptin therapy. There have been no spontaneous reports of opportunistic infections associated with saxagliptin use.
Vital Signs
Saxagliptin
No clinically meaningful changes in vital signs have been observed in patients treated with saxagliptin alone or in combination with metformin.
Laboratory Tests
Absolute Lymphocyte Counts
Saxagliptin
There was a dose-related mean decrease in absolute lymphocyte count observed with saxagliptin. From a baseline mean absolute lymphocyte count of approximately 2200 cells/microL, mean decreases of approximately 100 and 120 cells/microL with saxagliptin 5 mg and 10 mg, respectively, relative to placebo were observed at 24 weeks in a pooled analysis of five placebo-controlled clinical studies. Similar effects were observed when saxagliptin 5 mg and metformin were coadministered in treatment-naive patients compared to placebo and metformin. There was no difference observed for saxagliptin 2.5 mg relative to placebo. The proportion of patients who were reported to have a lymphocyte count ≤750 cells/microL was 0.5%, 1.5%, 1.4%, and 0.4% in the saxagliptin 2.5 mg, 5 mg, 10 mg, and placebo groups, respectively. In most patients, recurrence was not observed with repeated exposure to saxagliptin although some patients had recurrent decreases upon rechallenge that led to discontinuation of saxagliptin. The decreases in lymphocyte count were not associated with clinically relevant adverse reactions.
The clinical significance of this decrease in lymphocyte count relative to placebo is not known. When clinically indicated, such as in settings of unusual or prolonged infection, lymphocyte count should be measured. The effect of saxagliptin on lymphocyte counts in patients with lymphocyte abnormalities (e.g., human immunodeficiency virus) is unknown.
Platelets
Saxagliptin
Saxagliptin did not demonstrate a clinically meaningful or consistent effect on platelet count in the six, double-blind, controlled clinical safety and efficacy trials.
Vitamin B12 Concentrations
Metformin hydrochloride
Metformin may lower serum vitamin B12 concentrations. Measurement of hematologic parameters on an annual basis is advised in patients on KOMBIGLYZE XR and any apparent abnormalities should be appropriately investigated and managed. [See Warnings and Precautions (5.4).]
7 DRUG INTERACTIONS
7.1 Strong Inhibitors of CYP3A4/5 Enzymes
Saxagliptin
Ketoconazole significantly increased saxagliptin exposure. Similar significant increases in plasma concentrations of saxagliptin are anticipated with other strong CYP3A4/5 inhibitors (e.g., atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, and telithromycin). The dose of saxagliptin should be limited to 2.5 mg when coadministered with a strong CYP3A4/5 inhibitor. [See Dosage and Administration (2.2) and Clinical Pharmacology (12.3).]
7.2 Cationic Drugs
Metformin hydrochloride
Cationic drugs (e.g., amiloride, digoxin, morphine, procainamide, quinidine, quinine, ranitidine, triamterene, trimethoprim, or vancomycin) that are eliminated by renal tubular secretion theoretically have the potential for interaction with metformin by competing for common renal tubular transport systems. Such interaction between metformin and oral cimetidine has been observed in healthy volunteers. Although such interactions remain theoretical (except for cimetidine), careful patient monitoring and dose adjustment of KOMBIGLYZE XR and/or the interfering drug is recommended in patients who are taking cationic medications that are excreted via the proximal renal tubular secretory system.
7.3 Use with Other Drugs
Metformin hydrochloride
Some medications can predispose to hyperglycemia and may lead to loss of glycemic control. These medications include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel blockers, and isoniazid. When such drugs are administered to a patient receiving KOMBIGLYZE XR, the patient should be closely observed for loss of glycemic control. When such drugs are withdrawn from a patient receiving KOMBIGLYZE XR, the patient should be observed closely for hypoglycemia.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
There are no adequate and well-controlled studies in pregnant women with KOMBIGLYZE XR or its individual components. Because animal reproduction studies are not always predictive of human response, KOMBIGLYZE XR, like other antidiabetic medications, should be used during pregnancy only if clearly needed.
Coadministration of saxagliptin and metformin, to pregnant rats and rabbits during the period of organogenesis, was neither embryolethal nor teratogenic in either species when tested at doses yielding systemic exposures (AUC) up to 100 and 10 times the maximum recommended human doses (MRHD; saxagliptin 5 mg and metformin 2000 mg), respectively, in rats; and 249 and 1.1 times the MRHDs in rabbits. In rats, minor developmental toxicity was limited to an increased incidence of wavy ribs; associated maternal toxicity was limited to weight decrements of 11% to 17% over the course of the study, and related reductions in maternal food consumption. In rabbits, coadministration was poorly tolerated in a subset of mothers (12 of 30), resulting in death, moribundity, or abortion. However, among surviving mothers with evaluable litters, maternal toxicity was limited to marginal reductions in body weight over the course of gestation days 21 to 29; and associated developmental toxicity in these litters was limited to fetal body weight decrements of 7%, and a low incidence of delayed ossification of the fetal hyoid.
Saxagliptin
Saxagliptin was not teratogenic at any dose tested when administered to pregnant rats and rabbits during periods of organogenesis. Incomplete ossification of the pelvis, a form of developmental delay, occurred in rats at a dose of 240 mg/kg, or approximately 1503 and 66 times human exposure to saxagliptin and the active metabolite, respectively, at the MRHD of 5 mg. Maternal toxicity and reduced fetal body weights were observed at 7986 and 328 times the human exposure at the MRHD for saxagliptin and the active metabolite, respectively. Minor skeletal variations in rabbits occurred at a maternally toxic dose of 200 mg/kg, or approximately 1432 and 992 times the MRHD.
Saxagliptin administered to female rats from gestation day 6 to lactation day 20 resulted in decreased body weights in male and female offspring only at maternally toxic doses (exposures ≥1629 and 53 times saxagliptin and its active metabolite at the MRHD). No functional or behavioral toxicity was observed in offspring of rats administered saxagliptin at any dose.
Saxagliptin crosses the placenta into the fetus following dosing in pregnant rats.
Metformin hydrochloride
Metformin was not teratogenic in rats and rabbits at doses up to 600 mg/kg/day. This represents an exposure of about 2 and 6 times the maximum recommended human daily dose of 2000 mg based on body surface area comparisons for rats and rabbits, respectively. Determination of fetal concentrations demonstrated a partial placental barrier to metformin.
8.3 Nursing Mothers
No studies in lactating animals have been conducted with the combined components of KOMBIGLYZE XR. In studies performed with the individual components, both saxagliptin and metformin are secreted in the milk of lactating rats. It is not known whether saxagliptin or metformin are secreted in human milk. Because many drugs are secreted in human milk, caution should be exercised when KOMBIGLYZE XR is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of KOMBIGLYZE XR in pediatric patients have not been established.
8.5 Geriatric Use
KOMBIGLYZE XR
Elderly patients are more likely to have decreased renal function. Because metformin is contraindicated in patients with renal impairment, carefully monitor renal function in the elderly and use KOMBIGLYZE XR with caution as age increases. [See Warnings and Precautions (5.1, 5.2) and Clinical Pharmacology (12.3).]
Saxagliptin
In the six, double-blind, controlled clinical safety and efficacy trials of saxagliptin, 634 (15.3%) of the 4148 randomized patients were 65 years and over, and 59 (1.4%) patients were 75 years and over. No overall differences in safety or effectiveness were observed between patients ≥65 years old and the younger patients. While this clinical experience has not identified differences in responses between the elderly and younger patients, greater sensitivity of some older individuals cannot be ruled out.
Metformin hydrochloride
Controlled clinical studies of metformin did not include sufficient numbers of elderly patients to determine whether they respond differently from younger patients, although other reported clinical experience has not identified differences in responses between the elderly and young patients. Metformin is known to be substantially excreted by the kidney. Because the risk of lactic acidosis with metformin is greater in patients with impaired renal function, KOMBIGLYZE XR should only be used in patients with normal renal function. The initial and maintenance dosing of metformin should be conservative in patients with advanced age due to the potential for decreased renal function in this population. Any dose adjustment should be based on a careful assessment of renal function. [See Contraindications (4), Warnings and Precautions (5.2), and Clinical Pharmacology (12.3).]
10 OVERDOSAGE
Saxagliptin
In a controlled clinical trial, once-daily, orally-administered saxagliptin in healthy subjects at doses up to 400 mg daily for 2 weeks (80 times the MRHD) had no dose-related clinical adverse reactions and no clinically meaningful effect on QTc interval or heart rate.
In the event of an overdose, appropriate supportive treatment should be initiated as dictated by the patient’s clinical status. Saxagliptin and its active metabolite are removed by hemodialysis (23% of dose over 4 hours).
Metformin hydrochloride
Overdose of metformin hydrochloride has occurred, including ingestion of amounts greater than 50 grams. Hypoglycemia was reported in approximately 10% of cases, but no causal association with metformin hydrochloride has been established. Lactic acidosis has been reported in approximately 32% of metformin overdose cases [see Warnings and Precautions (5.1)]. Metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful for removal of accumulated drug from patients in whom metformin overdosage is suspected.
11 DESCRIPTION
KOMBIGLYZE XR (saxagliptin and metformin HCl extended-release) tablets contain two oral antihyperglycemic medications used in the management of type 2 diabetes: saxagliptin and metformin hydrochloride.
Saxagliptin
Saxagliptin is an orally-active inhibitor of the dipeptidyl-peptidase-4 (DPP4) enzyme.
Saxagliptin monohydrate is described chemically as (1S,3S,5S)-2-[(2S)-2-Amino-2-(3-hydroxytricyclo[3.3.1.13,7]dec-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile, monohydrate or (1S,3S,5S)-2-[(2S)-2-Amino-2-(3-hydroxyadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile hydrate. The empirical formula is C18H25N3O2•H2O and the molecular weight is 333.43. The structural formula is:
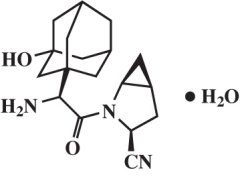
Saxagliptin monohydrate is a white to light yellow or light brown, non-hygroscopic, crystalline powder. It is sparingly soluble in water at 24°C ± 3°C, slightly soluble in ethyl acetate, and soluble in methanol, ethanol, isopropyl alcohol, acetonitrile, acetone, and polyethylene glycol 400 (PEG 400).
Metformin hydrochloride
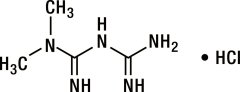
Metformin hydrochloride (N,N-dimethylimidodicarbonimidic diamide hydrochloride) is a white to off-white crystalline compound with a molecular formula of C4H11N5 • HCl and a molecular weight of 165.63. Metformin hydrochloride is freely soluble in water, slightly soluble in alcohol, and is practically insoluble in acetone, ether, and chloroform. The pKa of metformin is 12.4. The pH of a 1% aqueous solution of metformin hydrochloride is 6.68. The structural formula is:
KOMBIGLYZE XR
KOMBIGLYZE XR is available for oral administration as tablets containing either 5.58 mg saxagliptin hydrochloride (anhydrous) equivalent to 5 mg saxagliptin and 500 mg metformin hydrochloride (KOMBIGLYZE XR 5 mg/500 mg), or 5.58 mg saxagliptin hydrochloride (anhydrous) equivalent to 5 mg saxagliptin and 1000 mg metformin hydrochloride (KOMBIGLYZE XR 5 mg/1000 mg), or 2.79 mg saxagliptin hydrochloride (anhydrous) equivalent to 2.5 mg saxagliptin and 1000 mg metformin hydrochloride (KOMBIGLYZE XR 2.5 mg/1000 mg). Each film-coated tablet of KOMBIGLYZE XR contains the following inactive ingredients: carboxymethylcellulose sodium, hypromellose 2208, and magnesium stearate. The 5 mg/500 mg strength tablet of KOMBIGLYZE XR also contains microcrystalline cellulose and hypromellose 2910. In addition, the film coatings contain the following inactive ingredients: polyvinyl alcohol, polyethylene glycol 3350, titanium dioxide, talc, and iron oxides.
The biologically inert components of the tablet may occasionally remain intact during gastrointestinal transit and will be eliminated in the feces as a soft, hydrated mass.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
KOMBIGLYZE XR
KOMBIGLYZE XR combines two antihyperglycemic medications with complementary mechanisms of action to improve glycemic control in adults with type 2 diabetes: saxagliptin, a dipeptidyl-peptidase-4 (DPP4) inhibitor, and metformin hydrochloride, a biguanide.
Saxagliptin
Increased concentrations of the incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are released into the bloodstream from the small intestine in response to meals. These hormones cause insulin release from the pancreatic beta cells in a glucose-dependent manner but are inactivated by the DPP4 enzyme within minutes. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, reducing hepatic glucose production. In patients with type 2 diabetes, concentrations of GLP-1 are reduced but the insulin response to GLP-1 is preserved. Saxagliptin is a competitive DPP4 inhibitor that slows the inactivation of the incretin hormones, thereby increasing their bloodstream concentrations and reducing fasting and postprandial glucose concentrations in a glucose-dependent manner in patients with type 2 diabetes mellitus.
Metformin hydrochloride
Metformin improves glucose tolerance in patients with type 2 diabetes, lowering both basal and postprandial plasma glucose. Metformin decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. Unlike sulfonylureas, metformin does not produce hypoglycemia in patients with type 2 diabetes or in healthy subjects except in unusual circumstances [see Warnings and Precautions (5.8)] and does not cause hyperinsulinemia. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may actually decrease.
12.2 Pharmacodynamics
Saxagliptin
In patients with type 2 diabetes mellitus, administration of saxagliptin inhibits DPP4 enzyme activity for a 24-hour period. After an oral glucose load or a meal, this DPP4 inhibition resulted in a 2- to 3-fold increase in circulating levels of active GLP-1 and GIP, decreased glucagon concentrations, and increased glucose-dependent insulin secretion from pancreatic beta cells. The rise in insulin and decrease in glucagon were associated with lower fasting glucose concentrations and reduced glucose excursion following an oral glucose load or a meal.
Cardiac Electrophysiology
Saxagliptin
In a randomized, double-blind, placebo-controlled, 4-way crossover, active comparator study using moxifloxacin in 40 healthy subjects, saxagliptin was not associated with clinically meaningful prolongation of the QTc interval or heart rate at daily doses up to 40 mg (8 times the MRHD).
12.3 Pharmacokinetics
KOMBIGLYZE XR
Bioequivalence and food effect of KOMBIGLYZE XR was characterized under low calorie diet. The low calorie diet consisted of 324 kcal with meal composition that contained 11.1% protein, 10.5% fat, and 78.4% carbohydrate. The results of bioequivalence studies in healthy subjects demonstrated that KOMBIGLYZE XR combination tablets are bioequivalent to coadministration of corresponding doses of saxagliptin (ONGLYZA™) and metformin hydrochloride extended-release (GLUCOPHAGE® XR) as individual tablets under fed conditions.
Saxagliptin
The pharmacokinetics of saxagliptin and its active metabolite, 5-hydroxy saxagliptin were similar in healthy subjects and in patients with type 2 diabetes mellitus. The Cmax and AUC values of saxagliptin and its active metabolite increased proportionally in the 2.5 to 400 mg dose range. Following a 5 mg single oral dose of saxagliptin to healthy subjects, the mean plasma AUC values for saxagliptin and its active metabolite were 78 ng•h/mL and 214 ng•h/mL, respectively. The corresponding plasma Cmax values were 24 ng/mL and 47 ng/mL, respectively. The average variability (%CV) for AUC and Cmax for both saxagliptin and its active metabolite was less than 25%.
No appreciable accumulation of either saxagliptin or its active metabolite was observed with repeated once-daily dosing at any dose level. No dose- and time-dependence were observed in the clearance of saxagliptin and its active metabolite over 14 days of once-daily dosing with saxagliptin at doses ranging from 2.5 to 400 mg.
Metformin hydrochloride
Metformin extended-release Cmax is achieved with a median value of 7 hours and a range of 4 to 8 hours. At steady state, the AUC and Cmax are less than dose proportional for metformin extended-release within the range of 500 to 2000 mg. After repeated administration of metformin extended-release, metformin did not accumulate in plasma. Metformin is excreted unchanged in the urine and does not undergo hepatic metabolism. Peak plasma levels of metformin extended-release tablets are approximately 20% lower compared to the same dose of metformin immediate-release tablets, however, the extent of absorption (as measured by AUC) is similar between extended-release tablets and immediate-release tablets.
Absorption
Saxagliptin
The median time to maximum concentration (Tmax) following the 5 mg once daily dose was 2 hours for saxagliptin and 4 hours for its active metabolite. Administration with a high-fat meal resulted in an increase in Tmax of saxagliptin by approximately 20 minutes as compared to fasted conditions. There was a 27% increase in the AUC of saxagliptin when given with a meal as compared to fasted conditions. Saxagliptin may be administered with or without food. Food has no significant effect on the pharmacokinetics of saxagliptin when administered as KOMBIGLYZE XR combination tablets.
Metformin hydrochloride
Following a single oral dose of metformin extended-release, Cmax is achieved with a median value of 7 hours and a range of 4 to 8 hours. Although the extent of metformin absorption (as measured by AUC) from the metformin extended-release tablet increased by approximately 50% when given with food, there was no effect of food on Cmax and Tmax of metformin. Both high and low fat meals had the same effect on the pharmacokinetics of metformin extended-release. Food has no significant effect on the pharmacokinetics of metformin when administered as KOMBIGLYZE XR combination tablets.
Distribution
Saxagliptin
The in vitro protein binding of saxagliptin and its active metabolite in human serum is negligible. Therefore, changes in blood protein levels in various disease states (e.g., renal or hepatic impairment) are not expected to alter the disposition of saxagliptin.
Metformin hydrochloride
Distribution studies with extended-release metformin have not been conducted; however, the apparent volume of distribution (V/F) of metformin following single oral doses of immediate-release metformin 850 mg averaged 654 ± 358 L. Metformin is negligibly bound to plasma proteins, in contrast to sulfonylureas, which are more than 90% protein bound. Metformin partitions into erythrocytes, most likely as a function of time. Metformin is negligibly bound to plasma proteins and is, therefore, less likely to interact with highly protein-bound drugs such as salicylates, sulfonamides, chloramphenicol, and probenecid, as compared to the sulfonylureas, which are extensively bound to serum proteins.
Metabolism
Saxagliptin
The metabolism of saxagliptin is primarily mediated by cytochrome P450 3A4/5 (CYP3A4/5). The major metabolite of saxagliptin is also a DPP4 inhibitor, which is one-half as potent as saxagliptin. Therefore, strong CYP3A4/5 inhibitors and inducers will alter the pharmacokinetics of saxagliptin and its active metabolite. [See Drug Interactions (7.1).]
Metformin hydrochloride
Intravenous single-dose studies in healthy subjects demonstrate that metformin is excreted unchanged in the urine and does not undergo hepatic metabolism (no metabolites have been identified in humans) or biliary excretion.
Metabolism studies with extended-release metformin tablets have not been conducted.
Excretion
Saxagliptin
Saxagliptin is eliminated by both renal and hepatic pathways. Following a single 50 mg dose of 14C-saxagliptin, 24%, 36%, and 75% of the dose was excreted in the urine as saxagliptin, its active metabolite, and total radioactivity, respectively. The average renal clearance of saxagliptin (~230 mL/min) was greater than the average estimated glomerular filtration rate (~120 mL/min), suggesting some active renal excretion. A total of 22% of the administered radioactivity was recovered in feces representing the fraction of the saxagliptin dose excreted in bile and/or unabsorbed drug from the gastrointestinal tract. Following a single oral dose of saxagliptin 5 mg to healthy subjects, the mean plasma terminal half-life (t1/2) for saxagliptin and its active metabolite was 2.5 and 3.1 hours, respectively.
Metformin hydrochloride
Renal clearance is approximately 3.5 times greater than creatinine clearance, which indicates that tubular secretion is the major route of metformin elimination. Following oral administration, approximately 90% of the absorbed drug is eliminated via the renal route within the first 24 hours, with a plasma elimination half-life of approximately 6.2 hours. In blood, the elimination half-life is approximately 17.6 hours, suggesting that the erythrocyte mass may be a compartment of distribution.
Specific Populations
Renal Impairment
KOMBIGLYZE XR
In patients with decreased renal function (based on measured creatinine clearance), the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased in proportion to the decrease in creatinine clearance. Use of metformin in patients with renal impairment increases the risk for lactic acidosis. Because KOMBIGLYZE XR contains metformin, KOMBIGLYZE XR is contraindicated in patients with renal impairment [see Contraindications (4) and Warnings and Precautions (5.2)].
Hepatic Impairment
No pharmacokinetic studies of metformin have been conducted in patients with hepatic impairment. Use of metformin in patients with hepatic impairment has been associated with some cases of lactic acidosis. Because KOMBIGLYZE XR contains metformin, KOMBIGLYZE XR is not recommended in patients with hepatic impairment [see Warnings and Precautions (5.3)].
Body Mass Index
Saxagliptin
No dosage adjustment is recommended based on body mass index (BMI) which was not identified as a significant covariate on the apparent clearance of saxagliptin or its active metabolite in the population pharmacokinetic analysis.
Gender
Saxagliptin
No dosage adjustment is recommended based on gender. There were no differences observed in saxagliptin pharmacokinetics between males and females. Compared to males, females had approximately 25% higher exposure values for the active metabolite than males, but this difference is unlikely to be of clinical relevance. Gender was not identified as a significant covariate on the apparent clearance of saxagliptin and its active metabolite in the population pharmacokinetic analysis.
Metformin hydrochloride
Metformin pharmacokinetic parameters did not differ significantly between healthy subjects and patients with type 2 diabetes when analyzed according to gender (males=19, females=16). Similarly, in controlled clinical studies in patients with type 2 diabetes, the antihyperglycemic effect of metformin was comparable in males and females.
Geriatric
Saxagliptin
No dosage adjustment is recommended based on age alone. Elderly subjects (65-80 years of age) had 23% and 59% higher geometric mean Cmax and geometric mean AUC values, respectively, for saxagliptin than young subjects (18-40 years of age). Differences in active metabolite pharmacokinetics between elderly and young subjects generally reflected the differences observed in saxagliptin pharmacokinetics. The difference between the pharmacokinetics of saxagliptin and the active metabolite in young and elderly subjects is likely due to multiple factors including declining renal function and metabolic capacity with increasing age. Age was not identified as a significant covariate on the apparent clearance of saxagliptin and its active metabolite in the population pharmacokinetic analysis.
Metformin hydrochloride
Limited data from controlled pharmacokinetic studies of metformin in healthy elderly subjects suggest that total plasma clearance of metformin is decreased, the half-life is prolonged, and Cmax is increased, compared to healthy young subjects. From these data, it appears that the change in metformin pharmacokinetics with aging is primarily accounted for by a change in renal function.
KOMBIGLYZE XR should not be initiated in patients of any age unless measurement of creatinine clearance demonstrates that renal function is normal [see Warnings and Precautions (5.1, 5.2)].
Pediatric
Safety and effectiveness of KOMBIGLYZE XR in pediatric patients have not been established.
Race and Ethnicity
Saxagliptin
No dosage adjustment is recommended based on race. The population pharmacokinetic analysis compared the pharmacokinetics of saxagliptin and its active metabolite in 309 Caucasian subjects with 105 non-Caucasian subjects (consisting of six racial groups). No significant difference in the pharmacokinetics of saxagliptin and its active metabolite were detected between these two populations.
Metformin hydrochloride
No studies of metformin pharmacokinetic parameters according to race have been performed. In controlled clinical studies of metformin in patients with type 2 diabetes, the antihyperglycemic effect was comparable in whites (n=249), blacks (n=51), and Hispanics (n=24).
Drug Interactions
Specific pharmacokinetic drug interaction studies with KOMBIGLYZE XR have not been performed, although such studies have been conducted with the individual saxagliptin and metformin components.
In Vitro Assessment of Drug Interactions
In in vitro studies, saxagliptin and its active metabolite did not inhibit CYP1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, or 3A4, or induce CYP1A2, 2B6, 2C9, or 3A4. Therefore, saxagliptin is not expected to alter the metabolic clearance of coadministered drugs that are metabolized by these enzymes. Saxagliptin is a P-glycoprotein (P-gp) substrate, but is not a significant inhibitor or inducer of P-gp.
In Vivo Assessment of Drug Interactions
| Coadministered Drug | Dose of Coadministered Drug* | Dosing of Saxagliptin* | Geometric Mean Ratio (ratio with/without coadministered drug) No Effect = 1.00 |
||
|---|---|---|---|---|---|
| AUC† | Cmax | ||||
| * Single dose unless otherwise noted | |||||
| † AUC = AUC(INF) for drugs given as single dose and AUC = AUC(TAU) for drugs given in multiple doses | |||||
| ‡ Results exclude one subject | |||||
| ND=not determined; QD=once daily; q6h=every 6 hours; q12h=every 12 hours; BID=twice daily; LA=long acting | |||||
| No dosing adjustments required for the following: | |||||
| Metformin | 1000 mg | 100 mg | saxagliptin 5-hydroxy saxagliptin | 0.98 0.99 | 0.79 0.88 |
| Glyburide | 5 mg | 10 mg | saxagliptin 5-hydroxy saxagliptin | 0.98 ND | 1.08 ND |
| Pioglitazone‡ | 45 mg QD for 10 days | 10 mg QD for 5 days | saxagliptin 5-hydroxy saxagliptin | 1.11 ND | 1.11 ND |
| Digoxin | 0.25 mg q6h first day followed by q12h second day followed by QD for 5 days | 10 mg QD for 7 days | saxagliptin 5-hydroxy saxagliptin | 1.05 1.06 | 0.99 1.02 |
| Simvastatin | 40 mg QD for 8 days | 10 mg QD for 4 days | saxagliptin 5-hydroxy saxagliptin | 1.12 1.02 | 1.21 1.08 |
| Diltiazem | 360 mg LA QD for 9 days | 10 mg | saxagliptin 5-hydroxy saxagliptin | 2.09 0.66 | 1.63 0.57 |
| Rifampin | 600 mg QD for 6 days | 5 mg | saxagliptin 5-hydroxy saxagliptin | 0.24 1.03 | 0.47 1.39 |
| Omeprazole | 40 mg QD for 5 days | 10 mg | saxagliptin 5-hydroxy saxagliptin | 1.13 ND | 0.98 ND |
| Aluminum hydroxide + magnesium hydroxide + simethicone | aluminum hydroxide: 2400 mg magnesium hydroxide: 2400 mg simethicone: 240 mg | 10 mg | saxagliptin 5-hydroxy saxagliptin | 0.97 ND | 0.74 ND |
| Famotidine | 40 mg | 10 mg | saxagliptin 5-hydroxy saxagliptin | 1.03 ND | 1.14 ND |
| Limit KOMBIGLYZE XR dose to 2.5 mg/1000 mg once daily when coadministered with strong CYP3A4/5 inhibitors: | |||||
| Ketoconazole | 200 mg BID for 9 days | 100 mg | saxagliptin 5-hydroxy saxagliptin | 2.45 0.12 | 1.62 0.05 |
| Ketoconazole | 200 mg BID for 7 days | 20 mg | saxagliptin 5-hydroxy saxagliptin | 3.67 ND | 2.44 ND |
| Coadministered Drug | Dose of Coadministered Drug* | Dosing of Saxagliptin* | Geometric Mean Ratio (ratio with/without saxagliptin) No Effect = 1.00 |
||
|---|---|---|---|---|---|
| AUC† | Cmax | ||||
| * Single dose unless otherwise noted | |||||
| † AUC = AUC(INF) for drugs given as single dose and AUC = AUC(TAU) for drugs given in multiple doses | |||||
| ‡ Results include all subjects | |||||
| ND=not determined; QD=once daily; q6h=every 6 hours; q12h=every 12 hours; BID=twice daily; LA=long acting | |||||
| No dosing adjustments required for the following: | |||||
| Metformin | 1000 mg | 100 mg | metformin | 1.20 | 1.09 |
| Glyburide | 5 mg | 10 mg | glyburide | 1.06 | 1.16 |
| Pioglitazone‡ | 45 mg QD for 10 days | 10 mg QD for 5 days | pioglitazone hydroxy-pioglitazone | 1.08 ND | 1.14 ND |
| Digoxin | 0.25 mg q6h first day followed by q12h second day followed by QD for 5 days | 10 mg QD for 7 days | digoxin | 1.06 | 1.09 |
| Simvastatin | 40 mg QD for 8 days | 10 mg QD for 4 days | simvastatin simvastatin acid | 1.04 1.16 | 0.88 1.00 |
| Diltiazem | 360 mg LA QD for 9 days | 10 mg | diltiazem | 1.10 | 1.16 |
| Ketoconazole | 200 mg BID for 9 days | 100 mg | ketoconazole | 0.87 | 0.84 |
| Coadministered Drug | Dose of Coadministered Drug* | Dose of Metformin* | Geometric Mean Ratio (ratio with/without coadministered drug) No Effect = 1.00 |
||
|---|---|---|---|---|---|
| AUC† | Cmax | ||||
| * All metformin and coadministered drugs were given as single doses | |||||
| † AUC = AUC(INF) | |||||
| ‡ Ratio of arithmetic means | |||||
| No dosing adjustments required for the following: | |||||
| Glyburide | 5 mg | 850 mg | metformin | 0.91‡ | 0.93‡ |
| Furosemide | 40 mg | 850 mg | metformin | 1.09‡ | 1.22‡ |
| Nifedipine | 10 mg | 850 mg | metformin | 1.16 | 1.21 |
| Propranolol | 40 mg | 850 mg | metformin | 0.90 | 0.94 |
| Ibuprofen | 400 mg | 850 mg | metformin | 1.05‡ | 1.07‡ |
| Cationic drugs eliminated by renal tubular secretion may reduce metformin elimination: use with caution. [See Warnings and Precautions (5.9) and Drug Interactions (7.2).] | |||||
| Cimetidine | 400 mg | 850 mg | metformin | 1.40 | 1.61 |
| Coadministered Drug | Dose of Coadministered Drug* | Dose of Metformin* | Geometric Mean Ratio (ratio with/without metformin) No Effect = 1.00 |
||
|---|---|---|---|---|---|
| AUC† | Cmax | ||||
| * All metformin and coadministered drugs were given as single doses | |||||
| † AUC = AUC(INF) unless otherwise noted | |||||
| ‡ Ratio of arithmetic means, p-value of difference <0.05 | |||||
| § AUC(0-24 hr) reported | |||||
| ¶ Ratio of arithmetic means | |||||
| No dosing adjustments required for the following: | |||||
| Glyburide | 5 mg | 850 mg | glyburide | 0.78‡ | 0.63‡ |
| Furosemide | 40 mg | 850 mg | furosemide | 0.87‡ | 0.69‡ |
| Nifedipine | 10 mg | 850 mg | nifedipine | 1.10§ | 1.08 |
| Propranolol | 40 mg | 850 mg | propranolol | 1.01§ | 1.02 |
| Ibuprofen | 400 mg | 850 mg | ibuprofen | 0.97¶ | 1.01¶ |
| Cimetidine | 400 mg | 850 mg | cimetidine | 0.95§ | 1.01 |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
KOMBIGLYZE XR
No animal studies have been conducted with KOMBIGLYZE XR to evaluate carcinogenesis, mutagenesis, or impairment of fertility. The following data are based on the findings in the studies with saxagliptin and metformin individually.
Saxagliptin
Saxagliptin did not induce tumors in either mice (50, 250, and 600 mg/kg) or rats (25, 75, 150, and 300 mg/kg) at the highest doses evaluated. The highest doses evaluated in mice were equivalent to approximately 870 (males) and 1165 (females) times the human exposure at the MRHD of 5 mg/day. In rats, exposures were approximately 355 (males) and 2217 (females) times the MRHD.
Saxagliptin was not mutagenic or clastogenic with or without metabolic activation in an in vitro Ames bacterial assay, an in vitro cytogenetics assay in primary human lymphocytes, an in vivo oral micronucleus assay in rats, an in vivo oral DNA repair study in rats, and an oral in vivo/in vitro cytogenetics study in rat peripheral blood lymphocytes. The active metabolite was not mutagenic in an in vitro Ames bacterial assay.
In a rat fertility study, males were treated with oral gavage doses for 2 weeks prior to mating, during mating, and up to scheduled termination (approximately 4 weeks total) and females were treated with oral gavage doses for 2 weeks prior to mating through gestation day 7. No adverse effects on fertility were observed at exposures of approximately 603 (males) and 776 (females) times the MRHD. Higher doses that elicited maternal toxicity also increased fetal resorptions (approximately 2069 and 6138 times the MRHD). Additional effects on estrous cycling, fertility, ovulation, and implantation were observed at approximately 6138 times the MRHD.
Metformin hydrochloride
Long-term carcinogenicity studies have been performed in rats (dosing duration of 104 weeks) and mice (dosing duration of 91 weeks) at doses up to and including 900 mg/kg/day and 1500 mg/kg/day, respectively. These doses are both approximately 4 times the maximum recommended human daily dose of 2000 mg based on body surface area comparisons. No evidence of carcinogenicity with metformin was found in either male or female mice. Similarly, there was no tumorigenic potential observed with metformin in male rats. There was, however, an increased incidence of benign stromal uterine polyps in female rats treated with 900 mg/kg/day.
There was no evidence of a mutagenic potential of metformin in the following in vitro tests: Ames test (S. typhimurium), gene mutation test (mouse lymphoma cells), or chromosomal aberrations test (human lymphocytes). Results in the in vivo mouse micronucleus test were also negative.
Fertility of male or female rats was unaffected by metformin when administered at doses as high as 600 mg/kg/day, which is approximately 3 times the maximum recommended human daily dose based on body surface area comparisons.
13.2 Animal Toxicology
Saxagliptin
Saxagliptin produced adverse skin changes in the extremities of cynomolgus monkeys (scabs and/or ulceration of tail, digits, scrotum, and/or nose). Skin lesions were reversible at ≥20 times the MRHD but in some cases were irreversible and necrotizing at higher exposures. Adverse skin changes were not observed at exposures similar to (1-3 times) the MRHD of 5 mg. Clinical correlates to skin lesions in monkeys have not been observed in human clinical trials of saxagliptin.
14 CLINICAL STUDIES
There have been no clinical efficacy or safety studies conducted with KOMBIGLYZE XR to characterize its effect on hemoglobin A1c (A1C) reduction. Bioequivalence of KOMBIGLYZE XR with coadministered saxagliptin and metformin hydrochloride extended-release tablets has been demonstrated; however, relative bioavailability studies between KOMBIGLYZE XR and coadministered saxagliptin and metformin hydrochloride immediate-release tablets have not been conducted. The metformin hydrochloride extended-release tablets and metformin hydrochloride immediate-release tablets have a similar extent of absorption (as measured by AUC) while peak plasma levels of extended-release tablets are approximately 20% lower than those of immediate-release tablets at the same dose.
The coadministration of saxagliptin and metformin immediate-release tablets has been studied in adults with type 2 diabetes inadequately controlled on metformin alone and in treatment-naive patients inadequately controlled on diet and exercise alone. Treatment with saxagliptin dosed in the morning plus metformin immediate-release tablets at all doses produced clinically relevant and statistically significant improvements in hemoglobin A1c (A1C), fasting plasma glucose (FPG), and 2-hour postprandial glucose (PPG) following a standard oral glucose tolerance test (OGTT), compared to control. Reductions in A1C were seen across subgroups including gender, age, race, and baseline BMI.
Decrease in body weight in the treatment groups given saxagliptin in combination with metformin immediate-release was similar to that in the groups given metformin immediate-release alone. Saxagliptin plus metformin immediate-release was not associated with significant changes from baseline in fasting serum lipids compared to metformin alone.
In a 24-week, double-blind, randomized trial, patients treated with metformin immediate-release 500 mg twice daily for at least 8 weeks were randomized to continued treatment with metformin immediate-release 500 mg twice daily or to metformin extended-release either 1000 mg once daily or 1500 mg once daily. The mean change in A1C from baseline to Week 24 was 0.1% (95% confidence interval 0%, 0.3%) for the metformin immediate-release treatment arm, 0.3% (95% confidence interval 0.1%, 0.4%) for the 1000 mg metformin extended-release treatment arm, and 0.1% (95% confidence interval 0%, 0.3%) for the 1500 mg metformin extended-release treatment arm. Results of this trial suggest that patients receiving metformin immediate-release treatment may be safely switched to metformin extended-release once daily at the same total daily dose, up to 2000 mg once daily. Following a switch from metformin immediate-release to metformin extended-release, glycemic control should be closely monitored and dosage adjustments made accordingly.
Saxagliptin Morning and Evening Dosing
A 24-week monotherapy trial was conducted to assess a range of dosing regimens for saxagliptin. Treatment-naive patients with inadequately controlled diabetes (A1C ≥7% to ≤10%) underwent a 2-week, single-blind diet, exercise, and placebo lead-in period. A total of 365 patients were randomized to 2.5 mg every morning, 5 mg every morning, 2.5 mg with possible titration to 5 mg every morning, or 5 mg every evening of saxagliptin, or placebo. Patients who failed to meet specific glycemic goals during the study were treated with metformin rescue therapy added on to placebo or saxagliptin; the number of patients randomized per treatment group ranged from 71 to 74.
Treatment with either saxagliptin 5 mg every morning or 5 mg every evening provided significant improvements in A1C versus placebo (mean placebo-corrected reductions of −0.4% and −0.3%, respectively).
14.1 Coadministration of Saxagliptin with Metformin Immediate-Release in Treatment-Naive Patients
A total of 1306 treatment-naive patients with type 2 diabetes mellitus participated in this 24-week, randomized, double-blind, active-controlled trial to evaluate the efficacy and safety of saxagliptin coadministered with metformin immediate-release in patients with inadequate glycemic control (A1C ≥8% to ≤12%) on diet and exercise alone. Patients were required to be treatment-naive to be enrolled in this study.
Patients who met eligibility criteria were enrolled in a single-blind, 1-week, dietary and exercise placebo lead-in period. Patients were randomized to one of four treatment arms: saxagliptin 5 mg + metformin immediate-release 500 mg, saxagliptin 10 mg + metformin immediate-release 500 mg, saxagliptin 10 mg + placebo, or metformin immediate-release 500 mg + placebo (the maximum recommended approved saxagliptin dose is 5 mg daily; the 10 mg daily dose of saxagliptin does not provide greater efficacy than the 5 mg daily dose). Saxagliptin was dosed once daily. In the 3 treatment groups using metformin immediate-release, the metformin dose was up-titrated weekly in 500 mg per day increments, as tolerated, to a maximum of 2000 mg per day based on FPG. Patients who failed to meet specific glycemic goals during this study were treated with pioglitazone rescue as add-on therapy.
Coadministration of saxagliptin 5 mg plus metformin immediate-release provided significant improvements in A1C, FPG, and PPG compared with placebo plus metformin immediate-release (Table 7).
| Efficacy Parameter | Saxagliptin
5 mg + Metformin N=320 | Placebo + Metformin N=328 |
|---|---|---|
| * Intent-to-treat population using last observation on study or last observation prior to pioglitazone rescue therapy for patients needing rescue. | ||
| † Least squares mean adjusted for baseline value. | ||
| ‡ p-value <0.0001 compared to placebo + metformin | ||
| § p-value <0.05 compared to placebo + metformin | ||
| Hemoglobin A1C (%) | N=306 | N=313 |
| Baseline (mean) | 9.4 | 9.4 |
| Change from baseline (adjusted mean†) | −2.5 | −2.0 |
| Difference from placebo + metformin (adjusted mean†) | −0.5‡ | |
| 95% Confidence Interval | (−0.7, −0.4) | |
| Percent of patients achieving A1C <7% | 60%§ (185/307) | 41% (129/314) |
| Fasting Plasma Glucose (mg/dL) | N=315 | N=320 |
| Baseline (mean) | 199 | 199 |
| Change from baseline (adjusted mean†) | −60 | −47 |
| Difference from placebo + metformin (adjusted mean†) | −13§ | |
| 95% Confidence Interval | (−19, −6) | |
| 2-hour Postprandial Glucose (mg/dL) | N=146 | N=141 |
| Baseline (mean) | 340 | 355 |
| Change from baseline (adjusted mean†) | −138 | −97 |
| Difference from placebo + metformin (adjusted mean†) | −41§ | |
| 95% Confidence Interval | (−57, −25) | |
14.2 Addition of Saxagliptin to Metformin Immediate-Release
A total of 743 patients with type 2 diabetes participated in this 24-week, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of saxagliptin in combination with metformin immediate-release in patients with inadequate glycemic control (A1C ≥7% and ≤10%) on metformin alone. To qualify for enrollment, patients were required to be on a stable dose of metformin (1500-2550 mg daily) for at least 8 weeks.
Patients who met eligibility criteria were enrolled in a single-blind, 2-week, dietary and exercise placebo lead-in period during which patients received metformin immediate-release at their pre-study dose, up to 2500 mg daily, for the duration of the study. Following the lead-in period, eligible patients were randomized to 2.5 mg, 5 mg, or 10 mg of saxagliptin or placebo in addition to their current dose of open-label metformin immediate-release (the maximum recommended approved saxagliptin dose is 5 mg daily; the 10 mg daily dose of saxagliptin does not provide greater efficacy than the 5 mg daily dose). Patients who failed to meet specific glycemic goals during the study were treated with pioglitazone rescue therapy, added on to existing study medications. Dose titrations of saxagliptin and metformin immediate-release were not permitted.
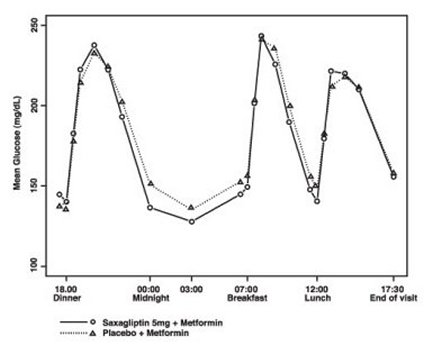
Saxagliptin 2.5 mg and 5 mg add-on to metformin immediate-release provided significant improvements in A1C, FPG, and PPG compared with placebo add-on to metformin immediate-release (Table 8). Mean changes from baseline for A1C over time and at endpoint are shown in Figure 1. The proportion of patients who discontinued for lack of glycemic control or who were rescued for meeting prespecified glycemic criteria was 15% in the saxagliptin 2.5 mg add-on to metformin immediate-release group, 13% in the saxagliptin 5 mg add-on to metformin immediate-release group, and 27% in the placebo add-on to metformin immediate-release group.
| Efficacy Parameter | Saxagliptin
2.5 mg + Metformin N=192 | Saxagliptin
5 mg + Metformin N=191 | Placebo + Metformin N=179 |
|---|---|---|---|
| * Intent-to-treat population using last observation on study or last observation prior to pioglitazone rescue therapy for patients needing rescue. | |||
| † Least squares mean adjusted for baseline value. | |||
| ‡ p-value <0.0001 compared to placebo + metformin | |||
| § p-value <0.05 compared to placebo + metformin | |||
| Hemoglobin A1C (%) | N=186 | N=186 | N=175 |
| Baseline (mean) | 8.1 | 8.1 | 8.1 |
| Change from baseline (adjusted mean†) | −0.6 | −0.7 | +0.1 |
| Difference from placebo (adjusted mean†) | −0.7‡ | −0.8‡ | |
| 95% Confidence Interval | (−0.9, −0.5) | (−1.0, −0.6) | |
| Percent of patients achieving A1C <7% | 37%§ (69/186) | 44%§ (81/186) | 17% (29/175) |
| Fasting Plasma Glucose (mg/dL) | N=188 | N=187 | N=176 |
| Baseline (mean) | 174 | 179 | 175 |
| Change from baseline (adjusted mean†) | −14 | −22 | +1 |
| Difference from placebo (adjusted mean†) | −16§ | −23§ | |
| 95% Confidence Interval | (−23, −9) | (−30, −16) | |
| 2-hour Postprandial Glucose (mg/dL) | N=155 | N=155 | N=135 |
| Baseline (mean) | 294 | 296 | 295 |
| Change from baseline (adjusted mean†) | −62 | −58 | −18 |
| Difference from placebo (adjusted mean†) | −44§ | −40§ | |
| 95% Confidence Interval | (−60, −27) | (−56, −24) | |
| * Includes patients with a baseline and week 24 value. Week 24 (LOCF) includes intent-to-treat population using last observation on study prior to pioglitazone rescue therapy for patients needing rescue. Mean change from baseline is adjusted for baseline value. |
|||
 |
|||
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
KOMBIGLYZE™ XR (saxagliptin and metformin HCl extended-release) tablets have markings on both sides and are available in the strengths and packages listed in Table 9.
| Tablet
Strength (saxagliptin and metformin HCl extended-release) | Film-Coated
Tablet Color/Shape | Tablet Markings | Package Size | NDC Code |
|---|---|---|---|---|
| 5 mg/500 mg | light brown to
brown, biconvex, capsule-shaped | “5/500” on one side and “4221” on the reverse, in blue ink | Bottles of 30 | 0003-4221-11 |
| 5 mg/1000 mg | pink, biconvex, capsule-shaped | “5/1000” on one side and “4223” on the reverse, in blue ink | Bottles of 30 Bottles of 90 Bottles of 500 | 0003-4223-11 0003-4223-21 0003-4223-31 |
| 2.5 mg/1000 mg | pale yellow
to light yellow, biconvex, capsule-shaped | “2.5/1000” on one side and “4222” on the reverse, in blue ink | Bottles
of 60 Bottles of 500 | 0003-4222-16 0003-4222-31 |
Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling.
17.1 Instructions
Patients should be informed of the potential risks and benefits of KOMBIGLYZE XR and of alternative modes of therapy. Patients should also be informed about the importance of adherence to dietary instructions, regular physical activity, periodic blood glucose monitoring and A1C testing, recognition and management of hypoglycemia and hyperglycemia, and assessment of diabetes complications. During periods of stress such as fever, trauma, infection, or surgery, medication requirements may change and patients should be advised to seek medical advice promptly.
The risks of lactic acidosis due to the metformin component, its symptoms and conditions that predispose to its development, as noted in Warnings and Precautions (5.1), should be explained to patients. Patients should be advised to discontinue KOMBIGLYZE XR immediately and to promptly notify their healthcare provider if unexplained hyperventilation, myalgia, malaise, unusual somnolence, dizziness, slow or irregular heart beat, sensation of feeling cold (especially in the extremities), or other nonspecific symptoms occur. Gastrointestinal symptoms are common during initiation of metformin treatment and may occur during initiation of KOMBIGLYZE XR therapy; however, patients should consult their physician if they develop unexplained symptoms. Although gastrointestinal symptoms that occur after stabilization are unlikely to be drug related, such an occurrence of symptoms should be evaluated to determine if it may be due to lactic acidosis or other serious disease.
Patients should be counseled against excessive alcohol intake while receiving KOMBIGLYZE XR.
Patients should be informed about the importance of regular testing of renal function and hematological parameters when receiving treatment with KOMBIGLYZE XR.
Patients should be informed that the incidence of hypoglycemia may be increased when KOMBIGLYZE XR is added to an insulin secretagogue (e.g., sulfonylurea).
Patients should be informed that KOMBIGLYZE XR must be swallowed whole and not crushed or chewed, and that the inactive ingredients may occasionally be eliminated in the feces as a soft mass that may resemble the original tablet.
Patients should be informed that if they miss a dose of KOMBIGLYZE XR, they should take the next dose as prescribed, unless otherwise instructed by their healthcare provider. Patients should be instructed not to take an extra dose the next day.
Physicians should instruct their patients to read the Patient Package Insert before starting KOMBIGLYZE XR therapy and to reread it each time the prescription is renewed. Patients should be instructed to inform their healthcare provider if they develop any unusual symptom or if any existing symptom persists or worsens.
GLUCOPHAGE® is a registered trademark of Merck Santé S.A.S., an associate of Merck KGaA
of Darmstadt, Germany. Licensed to Bristol-Myers Squibb Company.
ONGLYZA™ is a trademark of Bristol-Myers Squibb Company.
Manufactured by:
Bristol-Myers
Squibb Company
Princeton, NJ 08543 USA
Marketed
by:
Bristol-Myers Squibb Company
Princeton, NJ 08543
and
AstraZeneca
Pharmaceuticals LP
Wilmington, DE 19850
1281913
Iss
November 2010
PATIENT
INFORMATION
KOMBIGLYZE XR (kom-be-glyze X-R)
(saxagliptin and metformin
HCl extended-release)
tablets
Read the Patient Information that comes with KOMBIGLYZE XR before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is the most important information I should know about KOMBIGLYZE XR?
Serious side effects can happen in people taking KOMBIGLYZE XR, including:
Lactic Acidosis. Metformin hydrochloride, one of the medicines in KOMBIGLYZE XR, can cause a rare, but serious, side effect called lactic acidosis (a build-up of lactic acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in a hospital.
Stop taking KOMBIGLYZE XR and call your healthcare provider right away if you get any of the following symptoms of lactic acidosis:
- feel very weak and tired
- have unusual (not normal) muscle pain
- have trouble breathing
- have unusual sleepiness or sleep longer than usual
- have unexplained stomach or intestinal problems with nausea and vomiting, or diarrhea
- feel cold, especially in your arms and legs
- feel dizzy or lightheaded
- have a slow or irregular heartbeat
You have a higher chance of getting lactic acidosis if you:
- have kidney problems. People whose kidneys are not working properly should not take KOMBIGLYZE XR.
- have liver problems.
- have congestive heart failure that requires treatment with medicines.
- drink a lot of alcohol (very often or short-term “binge” drinking).
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids.
- have certain x-ray tests with injectable dyes or contrast agents.
- have surgery.
- have a heart attack, severe infection, or stroke.
- are 80 years of age or older and have not had your kidney function tested.
What is KOMBIGLYZE XR?
- KOMBIGLYZE XR is a prescription medicine that contains saxagliptin and metformin hydrochloride. KOMBIGLYZE XR is used with diet and exercise to help control high blood sugar (hyperglycemia) in adults with type 2 diabetes.
- KOMBIGLYZE XR is not for people with type 1 diabetes.
- KOMBIGLYZE XR is not for people with diabetic ketoacidosis (increased ketones in your blood or urine).
It is not known if KOMBIGLYZE XR is safe and effective in children younger than 18 years old.
Who should not take KOMBIGLYZE XR?
Do not take KOMBIGLYZE XR if you:
- have kidney problems.
- are allergic to the metformin hydrochloride in KOMBIGLYZE XR or any of the ingredients in KOMBIGLYZE XR. See the end of this leaflet for a complete list of ingredients in KOMBIGLYZE XR.
- have a condition called metabolic acidosis or diabetic ketoacidosis (increased ketones in your blood or urine).
- are going to get an injection of dye or contrast agents for an x-ray procedure or if you are going to have surgery and not able to eat or drink much. In these situations, KOMBIGLYZE XR will need to be stopped for a short time. Talk to your healthcare provider about when you should stop KOMBIGLYZE XR and when you should start KOMBIGLYZE XR again. See "What is the most important information I should know about KOMBIGLYZE XR?"
What should I tell my healthcare provider before taking KOMBIGLYZE XR?
Before you take KOMBIGLYZE XR, tell your healthcare provider if you:
- have type 1 diabetes. KOMBIGLYZE XR should not be used to treat people with type 1 diabetes.
- have a history or risk for diabetic ketoacidosis (high levels of certain acids, known as ketones, in the blood or urine). KOMBIGLYZE XR should not be used for the treatment of diabetic ketoacidosis.
- have kidney problems.
- have liver problems.
- have heart problems, including congestive heart failure.
- are older than 80 years. If you are over 80 years old you should not take KOMBIGLYZE XR unless your kidneys have been checked and they are normal.
- drink alcohol very often, or drink a lot of alcohol in short-term "binge" drinking.
- are taking insulin.
- have any other medical conditions.
- are pregnant or plan to become pregnant. It is not known if KOMBIGLYZE XR will harm your unborn baby. If you are pregnant, talk with your healthcare provider about the best way to control your blood sugar while you are pregnant.
- are breast-feeding or plan to breast-feed. It is not known if KOMBIGLYZE XR passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby while you take KOMBIGLYZE XR.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
KOMBIGLYZE XR may affect the way other medicines work, and other medicines may affect how KOMBIGLYZE XR works.
Tell your healthcare provider if you will be starting or stopping certain other types of medicines, such as antibiotics, or medicines that treat fungus or HIV/AIDS, because your dose of KOMBIGLYZE XR might need to be changed.
How should I take KOMBIGLYZE XR?
- Take KOMBIGLYZE XR exactly as your healthcare provider tells you.
- KOMBIGLYZE XR should be taken with meals to help lessen an upset stomach side effect.
- Swallow KOMBIGLYZE XR whole. Do not crush, cut, or chew KOMBIGLYZE XR.
- You may sometimes pass a soft mass in your stools (bowel movement) that looks like KOMBIGLYZE XR tablets.
- When your body is under some types of stress, such as fever, trauma (such as a car accident), infection, or surgery, the amount of diabetes medicine that you need may change. Tell your healthcare provider right away if you have any of these problems.
- Your healthcare provider should do blood tests to check how well your kidneys and liver are working before and during your treatment with KOMBIGLYZE XR.
- Your healthcare provider will check your diabetes with regular blood tests, including your blood sugar levels and your hemoglobin A1C.
- Follow your healthcare provider’s instructions for treating blood sugar that is too low (hypoglycemia). Talk to your healthcare provider if low blood sugar is a problem for you. See "What are the possible side effects of KOMBIGLYZE XR?"
- Check your blood sugar as your healthcare provider tells you to.
- Stay on your prescribed diet and exercise program while taking KOMBIGLYZE XR.
- If you miss a dose of KOMBIGLYZE XR, take your next dose as prescribed unless your healthcare provider tells you differently. Do not take an extra dose the next day.
- If you take too much KOMBIGLYZE XR, call your healthcare provider, local Poison Control Center, or go to the nearest hospital emergency room right away.
What are the possible side effects of KOMBIGLYZE XR?
KOMBIGLYZE XR can cause serious side effects, including:
- See "What is the most important information I should know about KOMBIGLYZE XR?"
-
Low blood sugar (hypoglycemia). If you take KOMBIGLYZE XR with another medicine that can cause low blood sugar, such as sulfonylureas or insulin, you have a higher risk of having low blood sugar. Tell your healthcare provider if you take other diabetes medicines. If you have symptoms of low blood sugar, you should check your blood sugar and treat if low, then call your healthcare provider. Symptoms of low blood sugar include:
- shaking
- sweating
- rapid heartbeat
- change in vision
- hunger
- headache
- change in mood
Common side effects of KOMBIGLYZE XR include:
- upper respiratory tract infection
- stuffy or runny nose and sore throat
- urinary tract infection
- headache
- diarrhea
- nausea and vomiting
Taking KOMBIGLYZE XR with meals can help lessen the common stomach side effects of metformin that usually happen at the beginning of treatment. If you have unexplained stomach problems, tell your healthcare provider. Stomach problems that start later during treatment may be a sign of something more serious.
Tell your healthcare provider if you have any side effects that bother you or that do not go away.
These are not all of the possible side effects of KOMBIGLYZE XR. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How should I store KOMBIGLYZE XR?
Store KOMBIGLYZE XR at 68°F to 77°F (20°C to 25°C).
Keep KOMBIGLYZE XR and all medicines out of the reach of children.
General information about the use of KOMBIGLYZE XR
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use KOMBIGLYZE XR for a condition for which it was not prescribed. Do not give KOMBIGLYZE XR to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about KOMBIGLYZE XR. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about KOMBIGLYZE XR that is written for healthcare professionals.
For more information, go to www.kombiglyzexr.com or call 1-800-664-5992.
What are the ingredients of KOMBIGLYZE XR?
Active ingredients: saxagliptin and metformin hydrochloride.
Inactive ingredients in each tablet: carboxymethylcellulose sodium, hypromellose 2208, and magnesium stearate.
The 5 mg/500 mg tablet also contains: microcrystalline cellulose and hypromellose 2910.
Tablet film coat contains: polyvinyl alcohol, polyethylene glycol 3350, titanium dioxide, talc, and iron oxides.
What is type 2 diabetes?
Type 2 diabetes is a condition in which your body does not make enough insulin, and the insulin that your body produces does not work as well as it should. Your body can also make too much sugar. When this happens, sugar (glucose) builds up in the blood. This can lead to serious medical problems.
The main goal of treating diabetes is to lower your blood sugar to a normal level.
High blood sugar can be lowered by diet and exercise, and by certain medicines when necessary.
Talk to your healthcare provider about how to prevent, recognize, and take care of low blood sugar (hypoglycemia), high blood sugar (hyperglycemia), and problems you have because of your diabetes.
KOMBIGLYZE XR (saxagliptin and metformin HCl extended-release)
tablets
Manufactured by:
Bristol-Myers Squibb
Company
Princeton, NJ 08543 USA
Marketed
by:
Bristol-Myers Squibb Company
Princeton, NJ 08543
and
AstraZeneca
Pharmaceuticals LP
Wilmington, DE 19850
1281913
Iss
November 2010
---------------------------------------------
REPRESENTATIVE PACKAGING
See How Supplied section for a complete list of available packages of KOMBIGLYZE XR.
30 Tablets
NDC 0003-4221-11
kombiglyze™ XR
(saxagliptin and metformin HCl extended-release) tablets
5 mg/500 mg
Rx only
Do not crush, cut, or chew tablets. Tablets must be swallowed whole.
Bristol-Myers Squibb
AstraZeneca
30 Tablets
NDC 0003-4223-11
kombiglyze™ XR
(saxagliptin and metformin HCl extended-release) tablets
5 mg/1000 mg
Rx only
Do not crush, cut, or chew tablets. Tablets must be swallowed whole.
Bristol-Myers Squibb
AstraZeneca
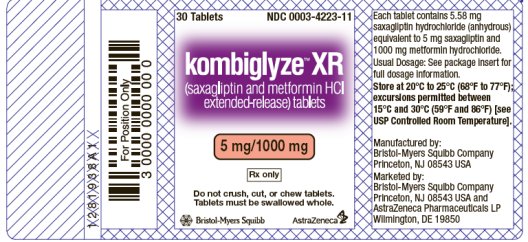
60 Tablets
NDC 0003-4222-16
kombiglyze™ XR
(saxagliptin and metformin HCl extended-release) tablets
2.5 mg/1000 mg
Rx only
Do not crush, cut, or chew tablets. Tablets must be swallowed whole.
Bristol-Myers Squibb
AstraZeneca
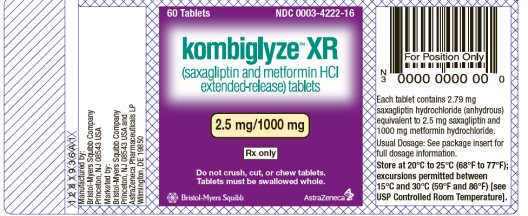
| KOMBIGLYZE XR
saxagliptin and metformin hydrochloride extended release tablet, film coated, extended release |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA200678 | 11/05/2010 | |
| KOMBIGLYZE XR
saxagliptin and metformin hydrochloride extended release tablet, film coated, extended release |
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA200678 | 11/05/2010 | |
| KOMBIGLYZE XR
saxagliptin and metformin hydrochloride extended release tablet, film coated, extended release |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA200678 | 11/05/2010 | |
| Labeler - E.R. Squibb & Sons, L.L.C. (011550092) |