MENTADENT SPECIALTY WHITENING TOOTHPASTE AND POLISHING
-
sodium monofluorophosphate and sodium fluoride
Church & Dwight Co., Inc.
----------
Drug Facts
Active ingredients
Whitening Polish Sodium monofluorophosphate 0.84%
Whitening Toothpaste Sodium Fluoride 0.24%
Purpose
Whitening Polish Anticavity toothpolish
Whitening Toothpaste Anticavity toothpaste
Use aids in the prevention of dental decay
Warnings
Keep out of reach of children under 6 years of age.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions do not swallow supervise children as necessary until capable of using without supervision
adults and children 2 years and older brush teeth thoroughly after meals or at least twice a day, or use as directed by a dentist or doctor
children under 6 years instruct in good brushing and rinsing habits (to minimize swallowing)
children under 2 years ask a dentist or physician
Whitening Polish
Inactive ingredients water, sorbitol, glycerin. calcium pyrophosphate, hydrated silica, aluminum hydroxide, dicalcium phosphate, flavor, PEG-12, sodium lauryl sulfate, cellulose gum, trisodium phosphate, sodium phosphate, sodium saccharin, titanium dioxide
Whitening Paste
Inactive ingredients sodium bicarbonate (baking soda), PEG-8, PEG/PPG-116/66 copolymer, sodium carbonate peroxide, tetrasodium pyrophosphate, silica, flavor, water, sodium saccharin, sodium lauroyl sarcosinate, sodium lauryl sulfate, polyethylene, sucralose, blue 1 lake
Questions or comments? Call 1-800-786-5135 Monday-Friday 9am-5pm ET
Mentadent
Speciality Whitening Toothpaste
and Polishing Kit
Clinically proven
to whiten teeth
- Helps protect surface enamel
- Strengthens teeth, and fights cavities
- Refreshing, clean taste
CONTENTS:
TOOTHPASTE Net wt. 3 oz. (85g)
TOOTHPOLISH Net wt. 3 oz. (85g)
Carton image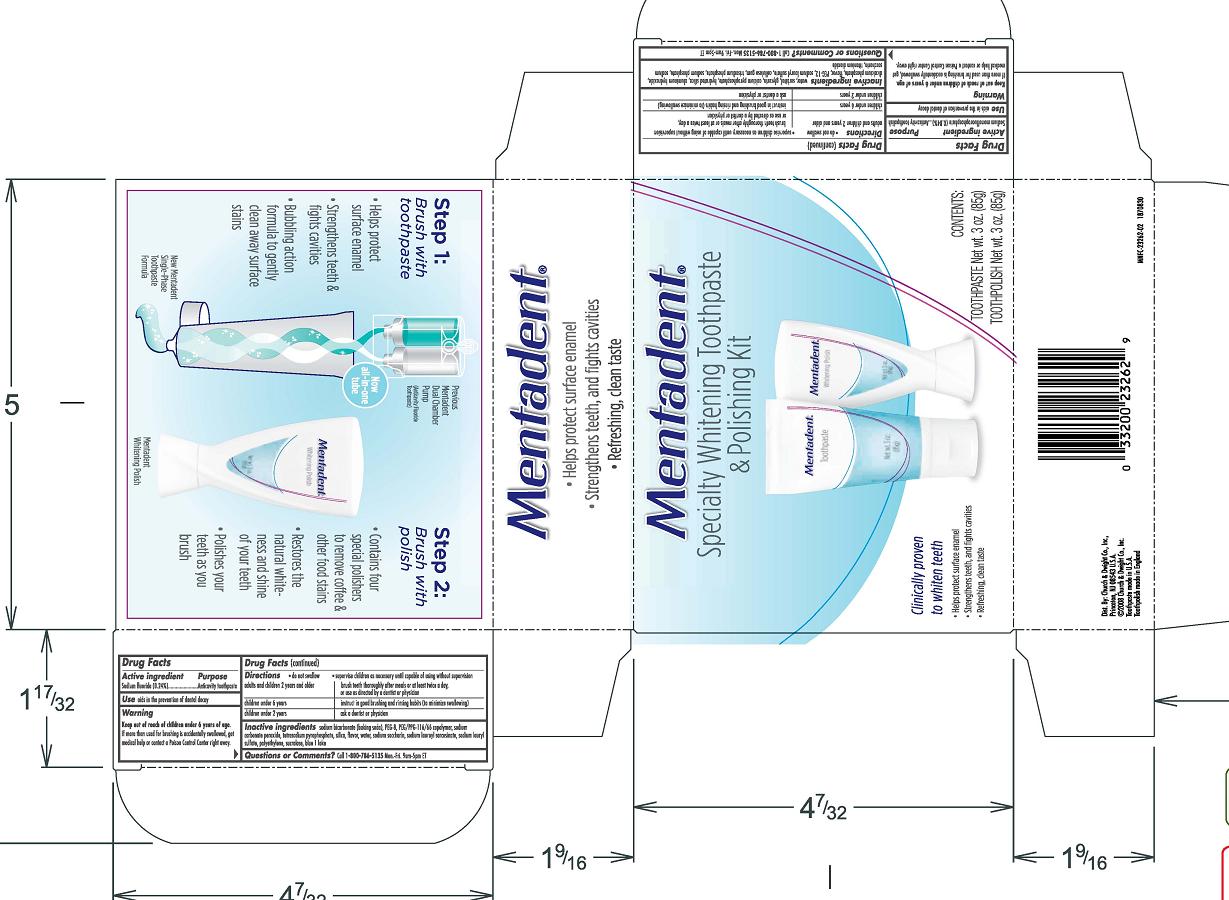
MENTADENT
SPECIALTY WHITENING TOOTHPASTE AND POLISHING
sodium monofluorophosphate and sodium fluoride
kit |
|
|
|
|
|
|
| Part 1 of 2 |
MENTADENT
TOOTHPOLISH
sodium monofluorophosphate
gel, dentifrice |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Part 2 of 2 |
MENTADENT
TOOTHPASTE
sodium fluoride
paste, dentifrice |
|
|
|
|
|
|
|
|
|
|
|
|
|
Revised: 11/2010Church & Dwight Co., Inc.
