CENTANY
-
mupirocin ointment
Medimetriks Pharmaceuticals Inc
----------
CENTANY®(mupirocin ointment, 2%)
For Dermatologic Use Only
Rx Only
DESCRIPTION
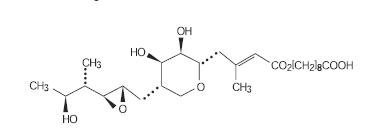
Each gram of CENTANY (mupirocin ointment, 2%) contains 20 mg mupirocin in a soft white ointment base consisting of caprylic/capric/myristic/stearic triglyceride, castor oil, oleyl alcohol, and propylene glycol monostearate. Mupirocin is a naturally occurring antibiotic. The chemical name is (E)-(2S,3R,4R,5S)-5-[(2S,3S,4S,5S)-2,3-Epoxy-5-hydroxy-4-methylhexyl]tetrahydro-3,4-dihydroxy-β-methyl-2H-pyran-2-crotonic acid, ester with 9-hydroxynonanoic acid. The molecular formula of mupirocin is C26H44O9 and the molecular weight is 500.62. The chemical structure is:
CLINICAL PHARMACOLOGY
Following the application of CENTANY (mupirocin ointment, 2%) to a 400cm2 area on the back of 23 healthy volunteers once daily for 7 days, the mean (range) cumulative urinary excretion of monic acid over 24 hrs following the last administration was 1.25% (0.2% to 3.0%) of the administered dose of mupirocin. The monic acid concentration in urine collected at specified intervals for 24 hrs on Day 7 ranged from <0.050 to 0.637 µg/mL.
Microbiology -
Mupirocin is an antibacterial agent produced by fermentation using the organism Pseudomonas fluorescens. Its spectrum of activity includes gram-positive bacteria. It is also active, in vitro only, against certain gram-negative bacteria. Mupirocin inhibits bacterial protein synthesis by reversibly and specifically binding to bacterial isoleucyl transfer-RNA synthetase. Due to this unique mode of action, mupirocin does not demonstrate cross-resistance with other classes of antimicrobial agents.
When mupirocin resistance occurs, it results from the production of a modified isoleucyl-tRNA synthetase or the acquisition, by genetic transfer, of a plasmid-mediating a new isoleucyl-tRNA synthetase. High-level plasmid-mediated resistance (MIC >500 mcg/mL) has been reported in increasing numbers of isolates of Staphylococcus aureus and with higher frequency in coagulase-negative staphylococci. Methicillin resistance and mupirocin resistance commonly occur together in Staphylococcus aureus and coagulase negative staphylococci.
Mupirocin is bactericidal at concentrations achieved by topical administration. However, the minimum bactericidal concentration (MBC) against relevant pathogens is generally eight-fold to thirty-fold higher than the minimum inhibitory concentration (MIC). In addition, mupirocin is highly protein bound (>97%), and the effect of wound secretions on the MICs of mupirocin has not been determined.
Mupirocin has been shown to be active against susceptible strains of Staphylococcus aureus and Streptococcus pyogenes, both in vitro and in clinical studies (see INDICATIONS AND USAGE).
INDICATIONS AND USAGE
CENTANY (mupirocin ointment, 2%) is indicated for the topical treatment of impetigo due to: Staphylococcus aureus and Streptococcus pyogenes.
CONTRAINDICATIONS
This drug is contraindicated in individuals with a history of sensitivity reactions to any of its components.
WARNINGS
CENTANY (mupirocin ointment, 2%) is not for ophthalmic use.
PRECAUTIONS
If a reaction suggesting sensitivity or chemical irritation should occur with the use of CENTANY (mupirocin ointment, 2%) treatment should be discontinued and appropriate alternative therapy for the infection instituted.
As with other antibacterial products, prolonged use may result in overgrowth of nonsusceptible organisms, including fungi. CENTANY (mupirocin ointment, 2%) is not formulated for use on mucosal surfaces. CENTANY (mupirocin ointment, 2%) is not intended for nasal use.
Information for Patients -
Use this medication only as directed by your healthcare provider. It is for external use only. Avoid contact with the eyes. The medication should be stopped and your healthcare practitioner contacted if irritation, severe itching or rash occurs. If impetigo has not improved in 3 to 5 days, contact your healthcare practitioner.
Drug Interactions -
The effect of the concurrent application of CENTANY (mupirocin ointment, 2%) and other drug products in unknown.
Carcinogenesis, Mutagenesis, Impairment of Fertility –
Long-term studies in animals to evaluate carcinogenic potential of mupirocin have not been conducted.
Results of the following studies performed with mupirocin calcium or mupirocin sodium in vitro and in vivo did not indicate a potential for genotoxicity: rat primary hepatocyte unscheduled DNA synthesis, sediment analysis for DNA strand breaks, Salmonella reversion test (Ames), Escherichia coli mutation assay, metaphase analysis of human lymphocytes, mouse lymphoma assay, and bone marrow micronuclei assay in mice.
Reproduction studies were performed in male and female rats with mupirocin administered subcutaneously at doses up to 14 times the human topical dose (approximately 60 mg mupirocin/day) on a mg/m2 basis and revealed neither evidence of impaired fertility nor impaired reproductive performance attributable to mupirocin.
Pregnancy
Teratogenic Effects
Pregnancy Category B
Reproduction studies have been performed in rats and rabbits with mupirocin administered subcutaneously at doses up to 22 and 43 times, respectively, the human topical dose (approximately 60 mg mupirocin per day) on a mg/m2 basis and revealed no evidence of harm to the fetus due to mupirocin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers -
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when CENTANY (mupirocin ointment, 2%) is administered to a nursing woman.
Pediatric Use -
The safety and effectiveness of CENTANY (mupirocin ointment, 2%) have been established in the age range of 2 months to 16 years. Use of CENTANY (mupirocin ointment, 2%) in these age groups is supported by evidence from adequate and well-controlled studies of CENTANY (mupirocin ointment, 2%) in impetigo in pediatric patients studied as a part of the pivotal clinical trials (see CLINICAL STUDIES).
ADVERSE REACTIONS
The following local adverse reactions have been reported in connection with the use of CENTANY (mupirocin ointment, 2%): application site reactions and pruritus, each in 1% of patients; contact dermatitis and furunculosis, each in 0.7% of patients; and exfoliative dermatitis and rash, each in 0.3% of patients.
DOSAGE AND ADMINISTRATION
A small amount of CENTANY (mupirocin ointment, 2%) should be applied to the affected area three times daily or as directed by a physician. The area treated may be covered with a gauze dressing if desired. Patients not showing a clinical response within 3 to 5 days should be re-evaluated.
CLINICAL STUDIES
The efficacy of topical CENTANY (mupirocin ointment, 2%) in impetigo was tested in one study. Patients with impetigo were randomized to receive either CENTANY (mupirocin ointment, 2%) or Bactroban® Ointment (mupirocin ointment, 2%) t.i.d. for 7 days. Clinical efficacy rates at the follow-up visit (one week after end of therapy) in the evaluable populations (adults and pediatric patients included) were 94% for CENTANY (mupirocin ointment, 2%) (n=233) and 95% for Bactroban® Ointment (mupirocin ointment, 2%) (n=242). Pathogen eradication rates at follow-up for both medications were 98%.
Pediatrics -
There were 413 pediatric patients aged 2 months to 15 years in the clinical study described above. Clinical efficacy rates at follow-up in the evaluable populations were 93% for CENTANY (mupirocin ointment, 2%) (n=199) and 95% for Bactroban® Ointment (mupirocin ointment, 2%) (n=214).
HOW SUPPLIED
CENTANY (mupirocin ointment, 2%) is available as follows:
30 g tube (NDC 43538-300-30)
Store at 20-25°C (68-77°F) [see USP Controlled Room Temperature].
Manufactured for Medimetriks Pharmaceuticals, Inc.
363 Route 46 West
Fairfield, NJ 07004-2402
www.medimetriks.com
Manufactured by PERRIGO
Allegan, MI 49010
U.S. Patent No. 6,013,657
Rev. 11/08 IP-006
Principal Display Panel - Carton
Centany®
(mupirocin ointment, 2%)
For Dermatologic Use Only
Rx Only
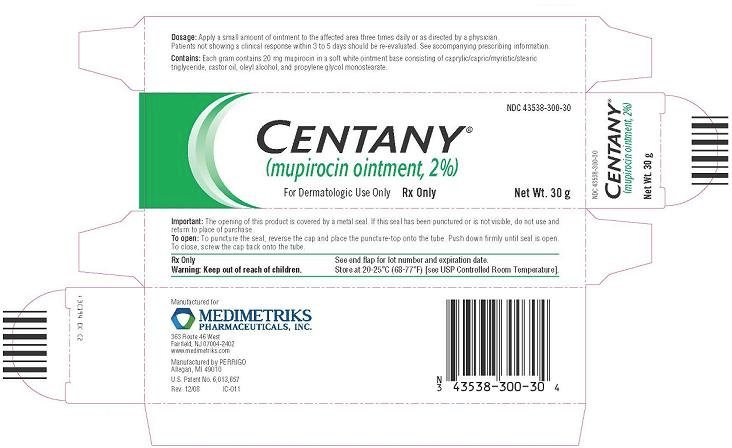
Centany(R) Carton
Principal Display Panel - Tube
Centany®
(mupirocin ointment, 2%)
For Dermatologic Use Only
Rx Only
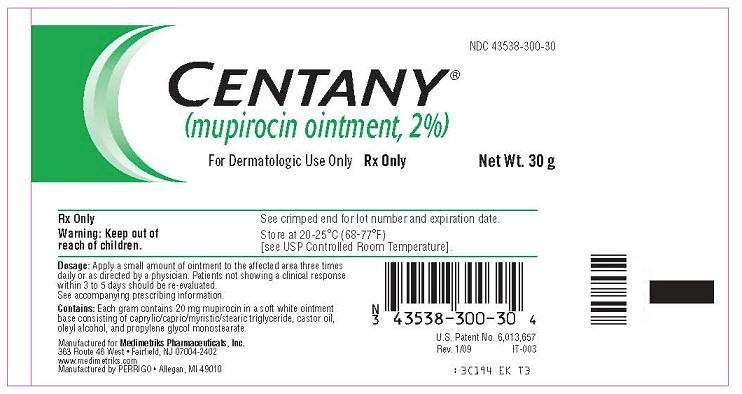
Centany(R) Tube
| CENTANY
mupirocin ointment |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA050788 | 02/26/2009 | |
| Labeler - Medimetriks Pharmaceuticals Inc (019903816) |