GUIATEX PE
-
guaifenesin and
phenylephrine hydrochloride liquid
Breckenridge Pharmaceutical, Inc.
----------
Guiatex PE™ SyrupDESCRIPTION
Each teaspoonful (5 mL) of Guiatex PE™ Syrup contains:
| Guaifenesin | 200 mg |
| Phenylephrine Hydrochloride | 5 mg |
INACTIVE INGREDIENTS
Sodium Benzoate, Citric Acid, Saccharin Sodium, Propylene Glycol, Polyethylene Glycol, Sorbitol Solution, Sucralose, D&C Red No. 33, FD&C Red No. 40, Strawberry Flavor, Purified Water.
Guaifenesin is an expectorant with the chemical name: 1,2-Propanediol, 3-(2-methoxyphenoxy)-,(±)-. Its structure is as follows:
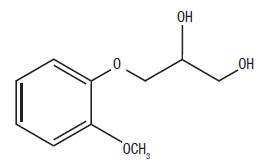
C10H14O4 M.W. 198.22
Phenylephrine Hydrochloride is a decongestant, having the chemical name (-)-m-Hydroxy-α-[(methylamino)methyl]benzyl alcohol hydrochloride. Its structure is as follows:
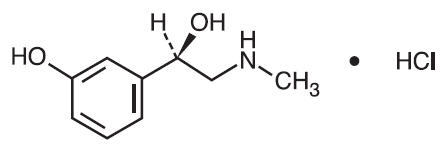
C9H13NO2 • HCl M.W. 203.67
CLINICAL PHARMACOLOGY
Guaifenesin
Guaifenesin is an expectorant which increases respiratory tract fluid secretions and helps to loosen phlegm and bronchial secretions. By reducing the viscosity of secretions, guaifenesin increases the efficiency of the cough reflex and of ciliary action in removing accumulated secretions from the trachea and bronchi. Guaifenesin is readily absorbed from the gastrointestinal tract and is rapidly metabolized and excreted in the urine. Guaifenesin has a plasma half-life of one hour. The major urinary metabolite is (α)-(2-methoxyphenoxy) lactic acid.
Phenylephrine Hydrochloride
Phenylephrine hydrochloride is a sympathomimetic amine which acts predominantly by a direct action on alpha (α) adrenergic receptors. In therapeutic doses, the drug has no significant stimulant effect on the beta (β) adrenergic receptors of the heart. Clinically, phenylephrine shrinks swollen mucous membranes, reduces tissue hyperemia, edema, and nasal congestion; and increases nasal airway patency. In therapeutic doses the drug causes little, if any, central nervous system (CNS) stimulation.
INDICATIONS AND USAGE
This product is indicated for temporarily relieving symptoms of upper respiratory tract disorders such as hay fever; as well as for the temporary relief of coughs associated with respiratory tract infections and related conditions such as pharyngitis, bronchitis, and asthma, when these conditions are complicated by tenacious mucus and/or mucus plugs and congestion. This product is effective in a productive as well as nonproductive cough, but is of particular value in a dry, nonproductive cough which tends to injure the mucous membrane of the air passages.
CONTRAINDICATIONS
This product is contraindicated in patients with hypersensitivity to guaifenesin or with hypersensitivity or idiosyncrasy to sympathomimetic amines which may be manifested by insomnia, dizziness, weakness, tremor or arrhythmias. Patients known to be hypersensitive to other sympathomimetic amines may exhibit cross sensitivity with phenylephrine. Phenylephrine is contraindicated in patients with hypertension or ventricular tachycardia and should be employed only with extreme caution in elderly patients or in patients with hyperthyroidism, bradycardia, partial heart block, myocardial disease, or severe arteriosclerosis. Phenylephrine is contraindicated in patients on monoamine oxidase inhibitor (MAOI) therapy and for 14 days after stopping MAOI therapy (see Drug Interactions).
WARNINGS
Do not exceed recommended dosage. Do not take this product for persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema, or where cough is accompanied by excessive phlegm (mucus) unless directed by a doctor. If nervousness, dizziness, or sleeplessness occurs, discontinue use and consult a doctor. If symptoms do not improve within 7 days or are accompanied by a fever, consult a doctor. Sympathomimetic amines should be used judiciously and sparingly in patients with hypertension, diabetes mellitus, ischemic heart disease, increased intraocular pressure, hyperthyroidism or prostatic hypertrophy. Sympathomimetics may produce central nervous system stimulation with convulsions or cardiovascular collapse with accompanying hypotension.
Hypertensive crisis can occur with concurrent use of phenylephrine and MAO inhibitors (and for 14 days after stopping MAOI therapy), indomethacin, or with beta (β) blockers and methyldopa. If a Hypertensive crisis occurs, these drugs should be discontinued immediately and therapy to lower blood pressure should be instituted. Fever should be managed by means of external cooling. Before prescribing medication to suppress or modify a cough, it is important that the underlying cause of the cough is identified, that modification of the cough does not increase the risk of physical or physiological complications, and that appropriate therapy for the primary disease is instituted.
PRECAUTIONS
Information for Patients
Patient consultation should include the following information regarding proper use of this medication:
- Do not take more medication than the amount recommended. Take medication with food, water, or milk to minimize gastric irritation.
- Do not take MAO inhibitors (or for 14 days after stopping MAOI therapy) while taking this medication.
- If a dose is missed, the medication should be taken as soon as possible unless it is almost time for the next dose; not doubling doses.
- This medication should be stored in a tight, light-resistant container at temperatures between 15°-30°C (59°-86°F).
- Keep this and all medications out of the reach of children. In case of accidental overdose, seek professional assistance or contact a poison control center immediately.
- Stimulants, such as phenylephrine, are banned and tested for by the U.S. Olympic Committee (USOC) and the National Collegiate Athletic Association (NCAA).
Drug Interactions
Beta (β) adrenergic blockers and MAO inhibitors (or for 14 days after stopping MAOI therapy) may potentiate the pressor effect of phenylephrine. Concurrent use of digitalis glycosides may increase the possibility of cardiac arrhythmias.
Sympathomimetics may reduce the hypotensive effects of guanethidine, mecamylamine, methyldopa, reserpine, and veratrum alkaloids. Concurrent use of tricyclic antidepressants may antagonize the effects of phenylephrine. Use of other vasopressor drugs during halothane anesthesia may cause serious cardiac arrhythmias.
Drug/Laboratory Test Interactions
Guaifenesin may increase renal clearance for urate and thereby lower serum uric acid levels. Guaifenesin may produce an increase in urinary 5-hydroxyindoleacetic acid and may therefore interfere with the interpretation of this test for the diagnosis of carcinoid syndrome. It may also falsely elevate the VMA test for catechols. Administration of the drug should be discontinued 48 hours prior to the collection of urine specimens for such tests.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No data are available on the long-term potential of Carcinogenesis, Mutagenesis or Impairment of Fertility in animals or humans.
Pregnancy
Pregnancy Category C
Animal reproduction studies have not been conducted with this drug. It is also not known whether this drug can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. This drug should be given to a pregnant woman only if clearly needed.
Nursing Mothers
Use of this product by nursing mothers is not recommended because of the higher than usual risk for an infant from sympathomimetic amines.
Pediatric Use
Safety and effectiveness in the pediatric population, under 6, have not been established. Appropriate studies on the relationship of age to the effects of Guaifenesin have not been performed in the pediatric population.
Geriatric Use
Geriatric patients are more likely to experience adverse reactions to sympathomimetics. Overdosage of sympathomimetics in this age group may cause hallucinations, convulsions, CNS depression, and death.
ADVERSE REACTIONS
Hyperreactive individuals may display ephedrine-like reactions such as tachycardia, palpitations, headache, dizziness or nausea. Sympathomimetics have been associated with certain untoward reactions including fear, anxiety, nervousness, restlessness, tremor, weakness, pallor, respiratory difficulty, dysuria, insomnia, hallucinations, convulsions, CNS depression, arrhythmias, and cardiovascular collapse with hypotension. No serious side effects from guaifenesin have been reported.
OVERDOSAGE
This product is comprised of pharmacologically different components (phenylephrine and guaifenesin). Therefore, it is difficult to predict the exact manifestation of symptoms in a given individual. A description of symptoms which are likely to appear after ingestion of an excess of the individual components follows: Overdosage with sympathomimetic amines can cause cardiac arrhythmias, cerebral hemorrhage and pulmonary edema. It can also cause palpitation, tremor, dizziness, vomiting, fear, labored breathing, headache, dryness of mouth, pallor, weakness, panic, anxiety, confusion, hallucinations, and delirium. Overdosage with guaifenesin is unlikely to produce toxic effects since its toxicity is low. Guaifenesin, when administered by stomach tube to test animals in doses up to 5 gm/kg, produced no signs of toxicity.
Treatment of acute overdosage would probably be based upon treating the patient for the symptoms of overdosage of phenylephrine as follows: The treatment of overdosage should provide symptomatic and supportive care. If the amount ingested is considered dangerous or excessive, induce vomiting with ipecac syrup unless the patient is convulsing, comatose, or has lost the gag reflex, in which case perform gastric lavage using a large bore tube. If indicated, follow with activated charcoal and a saline cathartic.
DOSAGE AND ADMINISTRATION
Adults and children over 12 years of age: 1 to 2 teaspoonfuls (5 to 10 mL) every 4 to 6 hours, not to exceed 12 teaspoons in 24 hours. Children 6 to 12 years: 1/2 to 1 teaspoonful (2.5 mL to 5 mL) every 4 to 6 hours, not to exceed 6 teaspoons in 24 hours. This product is not indicated for use in children under 6 years of age. (see PRECAUTIONS, Pediatric Use.). Shake well before using.
HOW SUPPLIED
Guiatex PE™ Syrup is supplied as a sugar free, alcohol free, strawberry flavored liquid, in bottles of 16 fl.oz. (473 mL), NDC 51991-597-16.
WARNING: KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Storage
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). See USP Controlled Room Temperature. Protect from freezing.
Pharmacist: Dispense in a tight, light-resistant container with a child-resistant closure as defined in the USP/NF.
All prescription substitutions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product.
Rx ONLY
Distributed by:
Breckenridge Pharmaceutical, Inc.
Boca Raton, FL 33487
Manufactured by:
Tri-Med Laboratories, Inc.
Somerset, NJ 08873
Iss. 7/08
PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
Breckenridge
Pharmaceutical, Inc.
NDC 51991-597-16
Guiatex PE™
Syrup
- Sugar Free
- Alcohol Free
Description: Each 5 mL (one teaspoonful) for oral
administration contains:
Guaifenesin 200 mg
Phenylephrine Hydrochloride 5 mg
INACTIVE INGREDIENTS: Sodium Benzoate, Citric
Acid, Saccharin Sodium, Propylene Glycol,
Polyethylene Glycol, Sorbitol Solution, Sucralose,
D&C Red No. 33, FD&C Red No. 40, Strawberry
Flavor, Purified Water.
Rx Only
Net Contents:
16 fl. oz. (473 mL)

| GUIATEX PE
guaifenesin and phenylephrine hydrochloride liquid |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| UNAPPROVED DRUG OTHER | 09/10/2008 | 01/31/2012 | |
| Labeler - Breckenridge Pharmaceutical, Inc. (150554335) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| TriMed Laboratories, Inc. | 182050567 | MANUFACTURE | |