CHLORPHENIRAMINE MALEATE
-
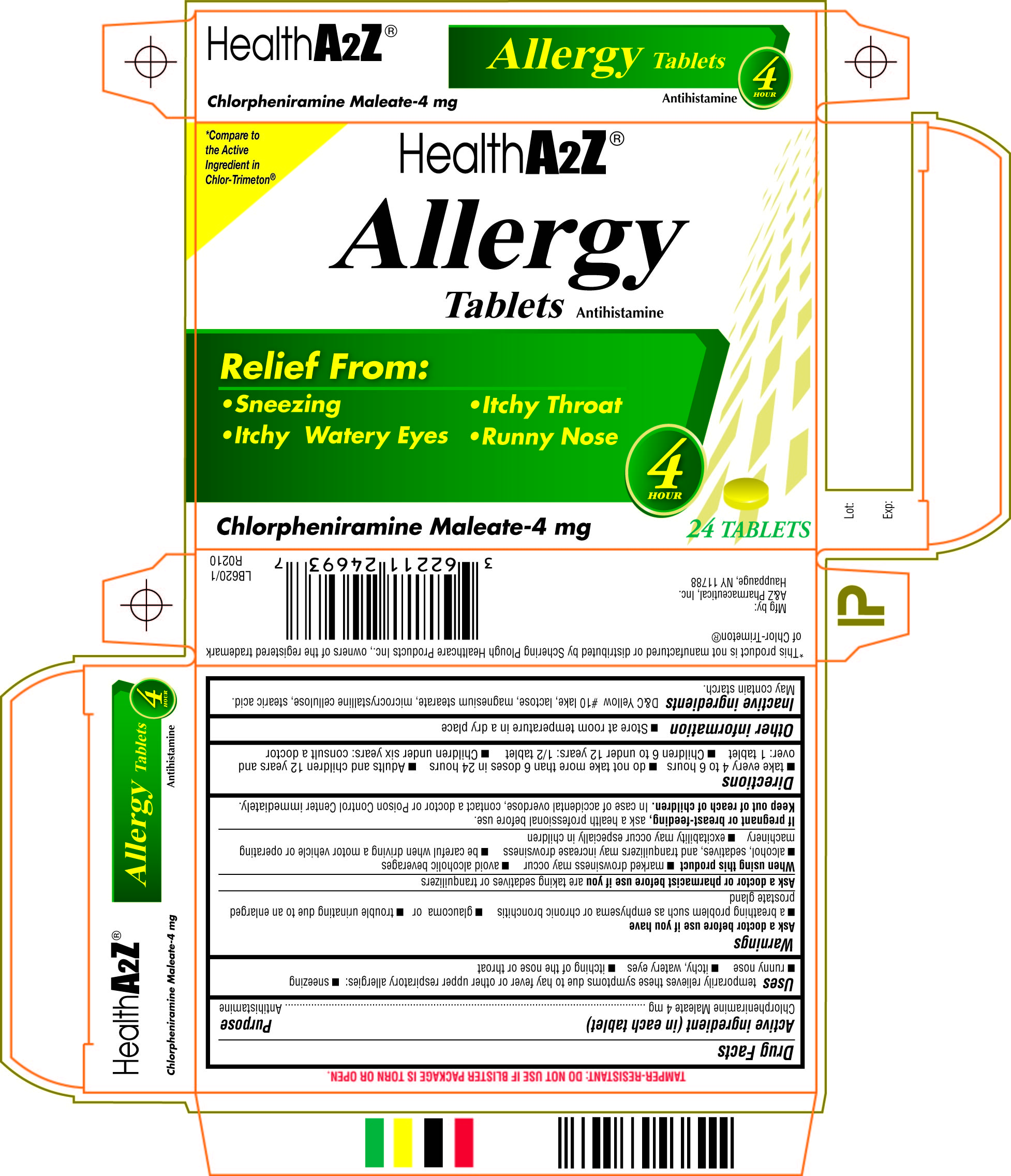
chlorpheniramine maleate tablet
A&Z Pharmaceutical, Inc.
----------
Chlorpheniramine maleateACTIVE INGREDIENT (IN EACH TABLET)
PURPOSE
USES
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: ■ sneezing ■ runny nose ■ itchy, watery eyes ■ itching of the nose or throat
WARNINGS
Ask a doctor before use if you have
■ a breathing problem such as emphysema or chronic bronchitis, ■ glaucoma or ■ trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you
are taking sedatives or tranquilizers
When using this product
■ marked drowsiness may occur ■ avoid alcoholic beverages ■ alcohol, sedatives, and tranquilizers may increase drowsiness ■ be careful when driving a motor vehicle or operating machinery ■ excitability may occur especially in children
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of accidental overdose, contact a doctor or Poison Control Center immediately.
Directions
■ take every 4 to 6 hours ■ do not take more than 6 doses in 24 hours ■ adults and children 12 years and over: 1 tablet ■ children 6 to under 12 years: 1/2 tablet ■ children under 6 years: consult a doctor
Other information
■ save carton for full directions and warnings ■ store at room temperature ■ do not use if imprinted safety seal under cap is broken or missing
Inactive ingredients
D.&C. Yellow #10 lake, lactose, magnesium stearate, microcrystalline cellulose, stearic acid. May contain starch.
DISPLAY PANEL
| CHLORPHENIRAMINE MALEATE
chlorpheniramine maleate tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 02/12/2010 | |
| Labeler - A&Z Pharmaceutical, Inc. (926820705) |
| Registrant - A&Z Pharmaceutical, Inc. (926820705) |