BIOSCRIPTIVES LIDUM
-
lidocaine hydrochloride cream
BioChemics, Inc
----------
Active Ingredients
Lidocaine 5%
Topical Anesthetic
Uses:
- rapid onset
- temporary relief of pain and itching due to
- minor burns
- skin irritations
- scrapes
- cuts
- sunburn
- insect bites
- for external use only
- in or near eyes
- in large quantities, particularly over raw surfaces or blistered areas
Stop use if:
- allergic reaction occurs
- condition worses or does not improve within 7 days
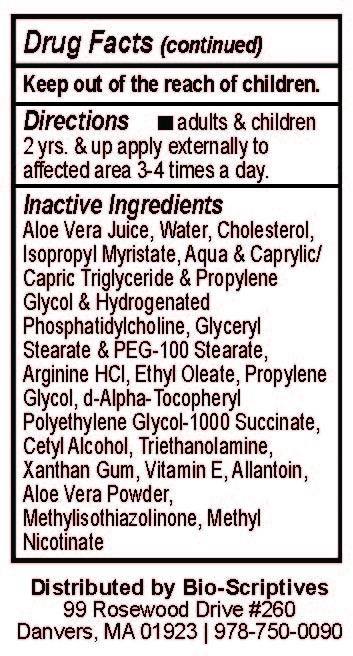
Keep out of the reach of children.
Directions: adults and children 2 years and up apply to externally to affected areas 3-4 times a day.
Inactive Ingredients: AloeVera Juice, Water, Cholesterol, lsopropyl Myristate, Aqua and Caprylic Capric Triglyceride and Propylene Glycol and Hydrogenated Phosphatitylcholine, Glyceryl Stearate and PEG-100 Stearate, ArgInIne HCI, Ethyl OLeate, Propylene Glycol, dAlpha-Tocopheryl Polyethylene Glycol-1000 SuccInate, Cetyl Alcohol, Triethanolamine, Xanthan Gum, Vitamin E, Allantoin, Aloe Vera Powder, Methylisothiazolinone, Methyl Nicotinate


| BIOSCRIPTIVES
LIDUM
lidocaine cream |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part348 | 07/23/2010 | |
| Labeler - BioChemics, Inc (802946426) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Coastal Products Company | 782445688 | manufacture | |