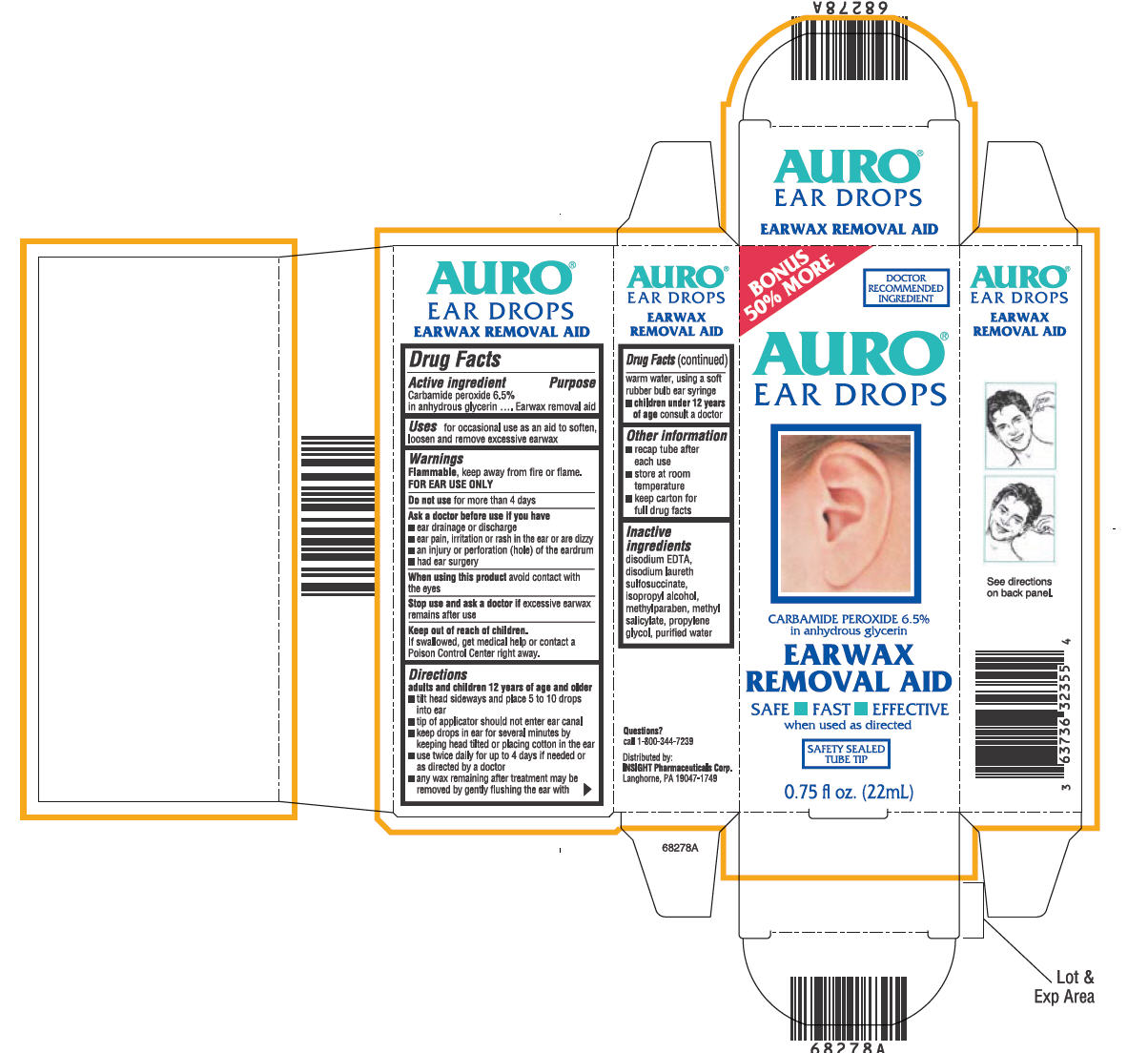
AURO EAR WAX REMOVER
-
carbamide peroxide liquid
Insight Pharmaceuticals LLC
----------
AURO®EAR DROPS
EARWAX REMOVAL AID
Drug Facts
Active ingredient
Carbamide peroxide 6.5% in anhydrous glycerin
Purpose
Earwax removal aid
Uses
for occasional use as an aid to soften, loosen and remove excessive earwax
Warnings
Flammable, keep away from fire or flame.
FOR EAR USE ONLY
Do not use for more than 4 days
Ask a doctor before use if you have
- ear drainage or discharge
- ear pain, irritation or rash in the ear or are dizzy
- an injury or perforation (hole) of the eardrum
- had ear surgery
When using this product avoid contact with the eyes
Stop use and ask a doctor if excessive earwax remains after use
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
adults and children 12 years of age and older
- tilt head sideways and place 5 to 10 drops into ear
- tip of applicator should not enter ear canal
- keep drops in ear for several minutes by keeping head tilted or placing cotton in the ear
- use twice daily for up to 4 days if needed or as directed by a doctor
- any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe
- children under 12 years of age consult a doctor
Other information
- recap tube after each use
- store at room temperature
- keep carton for full drug facts
Inactive ingredients
disodium EDTA, disodium laureth sulfosuccinate, isopropyl alcohol, methylparaben, methyl salicylate, propylene glycol, purified water
Questions?
call 1-800-344-7239
Distributed:
INSIGHT Pharmaceuticals Corp.
Langhorne, PA 19047-1749
PRINCIPAL DISPLAY PANEL - 22mL Carton
BONUS
50% MORE
DOCTOR
RECOMMENDED
INGREDIENT
AURO®
EAR DROPS
CARBAMIDE PEROXIDE 6.5%
in anhydrous glycerin
EARWAX
REMOVAL AID
SAFE ■ FAST ■ EFFECTIVE
when used as directed
SAFETY SEALED
TUBE TIP
0.75 fl oz. (22mL)
| AURO EAR WAX REMOVER
carbamide peroxide liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part344 | 07/01/2010 | |
| Labeler - Insight Pharmaceuticals LLC (176792315) |