CODEINE PHOSPHATE GUAIFENESIN
-
codeine phosphate and
guaifenesin liquid
Kylemore Pharmaceuticals, LLC
----------
Codeine Phosphate 10 mg Guaifenesin 200 mgDrug Facts
Active ingredients (in each 5 mL teaspoonful)
Codeine Phosphate* 10 mg ........ Antitussive
*WARNING: May be habit forming
Guaifenesin 200 mg ................. Expectorant
Uses
· temporarily relieves these symptoms due to the common cold:
· cough due to minor throat and bronchial irritation
· helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes and make coughs more productive
Warnings
Do not exceed recommended dosage.
Do not use this product
· do not take this product if you have chronic pulmonary disease, shortness of breath,or children who are taking other drugs, unless directed by a doctor.
Ask a doctor before use if you have
· a cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
· a cough that occurs with too much phlegm (mucus)
Stop use and ask a doctor if
· cough persists for more than 1 week, tends to recur, or is accompanied by a fever, rash or persistent headache. These could be signs of a serious condition.
· new symptoms occur
· may cause or aggravate constipation
If pregnant or breast-feeding, ask a health professional before use.
Keep out of the reach of children.
In case of accidental overdose, seek professional assistance, or contact a Poison Control Center immediately.
Directions
Take this medication with a full glass of water after each dose to help loosen mucus in the lungs.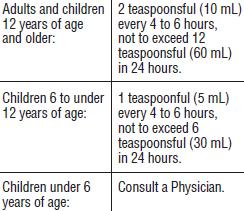
Inactive ingredients
Cherry Flavor, Citric Acid, Glycerin, MentholCrystals, Propylene Glycol, Purified Water,
Sodium Citrate, Sodium Saccharin, Sorbitol,
and Vanilla Flavor.
Other information
Store at 68° - 77° F (20° - 25° C)
Questions? Comments?
Call 1-866-934-6025

Kylemore Pharmaceuticals, LLC
Port St. Joe, FL 32456
323-10 Rev. 12/09
PACKAGING:
Codeine Phosphate 10 mg Guaifenesin 200 mg labeling:

| CODEINE PHOSPHATE GUAIFENESIN
codeine phosphate, guaifenesin liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 01/01/2010 | ||
| Labeler - Kylemore Pharmaceuticals, LLC (831892471) |