H.P. ACTHAR
-
corticotropin injection, solution
Questcor Pharmaceuticals, Inc.
----------
DESCRIPTION
H.P. Acthar® Gel (Repository Corticotropin Injection) is a highly purified sterile preparation of the adrenocorticotropic hormone in 16% gelatin to provide a prolonged release after intra-muscular or subcutaneous injection. Also contains 0.5% phenol, not more than 0.1% cysteine (added), sodium hydroxide and/or acetic acid to adjust pH and water for injection, q.s.
ACTH is a 39 amino acid peptide with the following chemical formula:
| H- | Ser- | Tyr- | Ser- | Met- | Glu- | His- | Phe- | Arg- | Trp- | Gly- | Lys- | Pro- | Val- | Gly- | |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| Lys- | Lys- | Arg- | Arg- | Pro- | Val- | Lys- | Val- | Try- | Pro- | Asp- | Gly- | Ala- | Glu- | Asp- | Gln- |
| 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| Leu- | Ala- | Glu- | Ala- | Phe- | Pro- | Leu- | Glu- | Phe- | OH | ||||||
| 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 |
CLINICAL PHARMACOLOGY
ACTH stimulates the adrenal cortex to secrete cortisol, corti¬costerone, aldosterone, and a number of weakly androgenic substances. Although ACTH does stimulate secretion of aldosterone, the rate is relatively independent. Prolonged administration of large doses of ACTH induces hyperplasia and hypertrophy of the adrenal cortex and continuous high output of cortisol, corticosterone and weak androgens. The release of ACTH is under the influence of the nervous system via the corticotropin regulatory hormone released from the hypothalamus and by a negative corticosteroid feedback mechanism. Elevated plasma cortisol suppresses ACTH release.
The trophic effects of ACTH on the adrenal cortex are not understood beyond the fact that they appear to be mediated by cyclic AMP.
ACTH rapidly disappears from the circulation following its intravenous administration; in man the plasma half-life is about 15 minutes.
The maximal effects of a trophic hormone on a target organ are achieved when optimal amounts of hormone are acting continuously. Thus, a fixed dose of ACTH will demonstrate a linear increase in adrenocortical secretion with increasing duration for the infusion.
INDICATIONS AND USAGE
H.P. Acthar Gel (Repository Corticotropin Injection) is indicated for diagnostic testing of adrenocortical function.
H.P. Acthar Gel (Repository Corticotropin Injection) has limited therapeutic value in those conditions responsive to corticosteroid therapy; in such cases, corticosteroid therapy is considered to be the treatment of choice. H.P. Acthar Gel (Repository Corticotropin Injection) may be employed in the following disorders:
ENDOCRINE DISORDERS: Nonsuppurative thyroiditis; Hypercalcemia associated with cancer.
NERVOUS SYSTEM DISEASES: Acute exacerbations of multiple sclerosis.
RHEUMATIC DISORDERS: As adjunctive therapy for short-term
administration (to tide the patient over an acute episode or
exacerbation) in:
Psoriatic arthritis; Rheumatoid arthritis,
including juvenile rheumatoid arthritis (selected cases may require
low-dose maintenance therapy); Ankylosing spondylitis; Acute and
subacute bursitis; Acute nonspecific tenosynovitis; Acute gouty
arthritis; Post-traumatic arthritis; Synovitis of osteoarthritis;
Epicondylitis.
COLLAGEN DISEASES: During an exacerbation or as maintenance therapy in selected cases of: Systemic lupus erythematosus; Systemic dermatomyositis (polymyositis); Acute rheumatic carditis.
DERMATOLOGIC DISEASES: Pemphigus; Bullous dermatitis herpetiformis; Severe erythema multiforme (Stevens-Johnson syndrome); Exfoliative dermatitis; Severe psoriasis; Severe seborrheic dermatitis; Mycosis fungoides.
ALLERGIC STATES: Control of severe or incapacitating allergic
conditions intractable to adequate trials of conventional
treatment:
Seasonal or perennial allergic rhinitis; Bronchial
asthma; Contact dermatitis; Atopic dermatitis; Serum sickness.
OPHTHALMIC DISEASES: Severe acute and chronic allergic and
inflammatory processes involving the eye and its adnexa such as:
Allergic conjunctivitis; Keratitis; Herpes zoster ophthalmicus; Iritis
and iridocyclitis; Diffuse posterior uveitis and choroiditis; Optic
neuritis; Sympathetic ophthalmia; Chorioretinitis; Anterior segment
inflammation; Allergic corneal marginal ulcers.
RESPIRATORY DISEASES: Symptomatic sarcoidosis; Loeffler's syndrome not manageable by other means; Berylliosis; Fulminating or disseminated pulmonary tuberculosis when used concurrently with antituberculous chemotherapy; Aspiration pneumonitis.
HEMATOLOGIC DISORDERS: Acquired (autoimmune) hemolytic anemia; Secondary thrombocytopenia in adults; Erythro-blastopenia (RBC anemia); Congenital (erythroid) hypoplastic anemia.
NEOPLASTIC DISEASES: For palliative management of: Leukemias and lymphomas in adults; Acute leukemia of childhood.
EDEMATOUS STATE: To induce a diuresis or a remission of proteinuria in the nephrotic syndrome without uremia of the idiopathic type or that due to lupus erythematosus.
GASTROINTESTINAL DISEASES: To tide the patient over a critical period of the disease in: Ulcerative colitis; Regional enteritis.
MISCELLANEOUS: Tuberculous meningitis with subarachnoid block or impending block when used concurrently with appropriate antituberculous chemotherapy; Trichinosis with neurologic or myocardial involvement.
CONTRAINDICATIONS
Corticotropin is contraindicated in patients with scleroderma, osteoporosis, systemic fungal infections, ocular herpes simplex, recent surgery, history of or the presence of a peptic ulcer, congestive heart failure, hypertension, or sensitivity to proteins of porcine origin.
Treatment of conditions listed within the INDICATIONS section (see above) is contraindicated when they are accompanied by primary adrenocortical insufficiency or adrenocortical hyperfunction.
Intravenous administration of corticotropin is contraindicated.
WARNINGS
Chronic administration of corticotropin may lead to adverse effects which are not reversible. Corticotropin may only suppress symptoms and signs of chronic diseases without altering the natural course of the disease. H.P. Acthar Gel (Repository Corticotropin Injection) should not be administered for treatment until adrenal responsiveness has been verified with the route of administration which will be utilized during treatment, intramuscularly or subcutaneously. A rise in urinary and plasma corticosteroid values provides direct evidence of a stimulatory effect. Prolonged administration of corticotropin increases the risk of hypersensitivity reactions. Although the action of corticotropin is similar to that of exogenous adrenocortical steroids, the quantity of adrenocorticoid may be variable. In patients who receive prolonged corticotropin therapy, the additional use of rapidly acting corticosteroids before, during, and after an unusual stressful situation is indicated.
Prolonged use of corticotropin may produce posterior subcapsular cataracts and glaucoma with possible damage to the optic nerves.
Corticotropin may mask some signs of infection, and new infections including those of the eye due to fungi or viruses may appear during its use. There may be decreased resistance and inability to localize infection when corticotropin is used.
Corticotropin can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium. Dietary salt restriction and potassium supplementation may be necessary. Corticotropin increases calcium excretion.
While on corticotropin therapy, patients should not be vaccinated against smallpox. Other immunization procedures should be undertaken with caution in patients who are receiving corticotropin, especially when high doses are administered because of the possible hazards of neurological complications and lack of antibody response.
PRECAUTIONS
1. General
Corticotropin injection should be used in the lowest dose for the shortest period of time to accomplish the therapeutic goal. Corticotropin should be used for treatment only when the disease is intractable to non-steroid treatment.
There is an enhanced effect in patients with hypothyroidism and in those with cirrhosis of the liver. Sensitivity to porcine protein should be considered before starting therapy and during the course of treatment should symptoms arise.
When an infection is present, appropriate antibiotic therapy should be given. Patients with latent tuberculosis should be observed closely, and if therapy is prolonged, chemoprophylaxis should be instituted.
Psychic symptoms may appear with use of corticotropin or pre-existing symptoms may be enhanced. These may range from mood alteration to a psychotic state.
Patients with a secondary disease may have that disease worsened. Caution should be used when prescribing corticotropin in patients with diabetes, renal insufficiency, diverticulitis, and myasthenia gravis.
Corticotropin often acts by suppressing symptoms without altering the course of the underlying disease. Since complications with corticotropin use are dependent on the dose and duration of treatment, a risk/benefit decision must be made in each case.
Suppression of the pituitary adrenal axis occurs following prolonged therapy which may be slow in returning to normal. Patients should be protected from the stress of trauma or surgery by the use of corticosteroids during the period of stress.
Since maximal corticotropin stimulation of the adrenals may be limited during the first few days of treatment, other drugs should be administered when an immediate therapeutic effect is desirable.
Although controlled clinical trials have shown ACTH to be effective in speeding the resolution of acute exacerbations of multiple sclerosis, they do not show that it affects the ultimate outcome or natural history of the disease. The studies do show that relatively high doses of ACTH are necessary to demonstrate a significant effect. (See DOSAGE AND ADMINISTRATION section).
Treatment of acute gouty arthritis should be limited to a few days. Since rebound attacks may occur when corticotropin is discontinued, conventional concomitant therapy should be administered during corticotropin treatment and for several days after it is stopped.
Aspirin should be used cautiously in conjunction with corticotropin in hypoprothrombinemia.
2. Drug Interactions:
Corticotropin may accentuate the electrolyte loss associated with diuretic therapy.
3. Carcinogenesis, Mutagenesis, Impairment of Fertility:
Adequate and well-controlled studies have not been done in animals. Human use has not been associated with an increase in malignant disease. See Pregnancy warning below.
4. Pregnancy
Pregnancy Class C: Corticotropin has been shown to have an embryocidal effect. There are no adequate and well-controlled studies in pregnant women. Corticotropin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
5. Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from corticotropin, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
6. Pediatric Use
Prolonged use of corticotropin in children will inhibit skeletal growth. If use is necessary, it should be given intermittently and the child carefully observed.
ADVERSE REACTIONS
Fluid and electrolyte disturbances:
Sodium retention; fluid
retention; potassium loss; hypokalemic alkalosis; calcium loss.
Musculoskeletal:
Muscle weakness; steroid myopathy; loss of
muscle mass; osteoporosis; vertebral compression fractures; aseptic
necrosis of femoral and humeral heads; pathologic fracture of long
bones.
Gastrointestinal:
Peptic ulcer with possible perforation and
hemorrhage; pancreatitis; abdominal distention; ulcerative esophagitis.
Dermatologic:
Impaired wound healing; thin fragile skin;
petechiae and ecchymoses; facial erythema; increased sweating;
suppression of skin test reactions; acne; hyperpigmentation.
Cardiovascular:
Hypertension; necrotizing angiitis;
congestive heart failure
Neurological:
Convulsions; increased intracranial pressure
with papilledema, (pseudo-tumor cerebri) usually after treatment;
headache; vertigo.
Endocrine:
Menstrual irregularities; development of
Cushingoid state; suppression of growth in children; secondary
adrenocortical and pituitary unresponsiveness, particularly in times of
stress, as in trauma, surgery or illness; decreased carbohydrate
tolerance; manifestations of latent diabetes mellitus; increased
requirements for insulin or oral hypoglycemic agents in diabetics;
hirsutism.
Ophthalmic:
Posterior subcapsular cataracts; increased
intraocular pressure; glaucoma with possible damage to optic nerve;
exophthalmos.
Metabolic:
Negative nitrogen balance due to protein
catabolism.
Allergic reactions:
Especially in patients with allergic
responses to proteins manifesting as dizziness, nausea and vomiting,
shock, skin reactions.
Miscellaneous:
Abscess; prolonged use of ACTH may result in
antibodies to it and resulting loss of stimulatory effect.
Drug Abuse and Dependence
Although drug dependence does not occur, sudden withdrawal of corticotropin after prolonged use may lead to recurrent symptoms which make it difficult to stop. It may be necessary to taper the dose and increase the injection interval to gradually discontinue the medication.
OVERDOSAGE
An acute overdose would present no different adverse reactions.
DOSAGE AND ADMINISTRATION
Standard tests for verification of adrenal responsiveness to corticotropin may utilize as much as 80 units as a single injection or one or more injections of a lesser dosage. Verification tests should be performed prior to treatment with corticotropins. The test should utilize the route(s) of administration proposed for treatment. Following verification, dosage should be individualized according to the disease under treatment and the general medical condition of each patient. Frequency and dose of the drug should be determined by considering severity of the disease, plasma and urine corticosteroid levels and the initial response of the patient. Only gradual change in dosage schedules should be attempted, after full drug effects have become apparent.
The chronic administration of more than 40 units daily may be associated with uncontrollable adverse effects.
When reduction in dosage is indicated this should be done gradually by either reducing the amount of each injection, administering injections at longer intervals or by a combination of both of the above. During reduction of dosage careful consideration should be given to the disease being treated, the general medical conditions of the patient and the duration over which corticotropin was administered.
The usual dose of H.P. Acthar® Gel (Repository Corticotropin Injection) is 40-80 units given intramuscularly or subcutaneously every 24-72 hours.
In the treatment of acute exacerbations of multiple sclerosis daily intramuscular doses of 80-120 units for 2-3 weeks may be administered.
Preparation: H.P. Acthar Gel (Repository Corticotropin Injection) should be warmed to room temperature before using.
Caution: Do not over pressurize the vial prior to withdrawing the product.
How Supplied:
| Presentation | NDC | |
| 5 mL multi-dose vial containing 80 USP Units per mL | 63004-7731-1 |
Storage:
Store H.P. Acthar Gel (Repository Corticotropin Injection) under refrigeration between 2°-8°C (36°-46°F).
Product is stable for the period indicated on the label when stored under the conditions described.
Rev. 10/01 No. 1350 PL065/Rev.01
Manufactured for Questcor Pharmaceuticals, Inc.
Questcor Pharmaceuticals, Inc.
3260 Whipple Road
Union
City, CA 94597 USA
phone (800) 411-3065
(510) 400-0700
fax (510) 400-0799
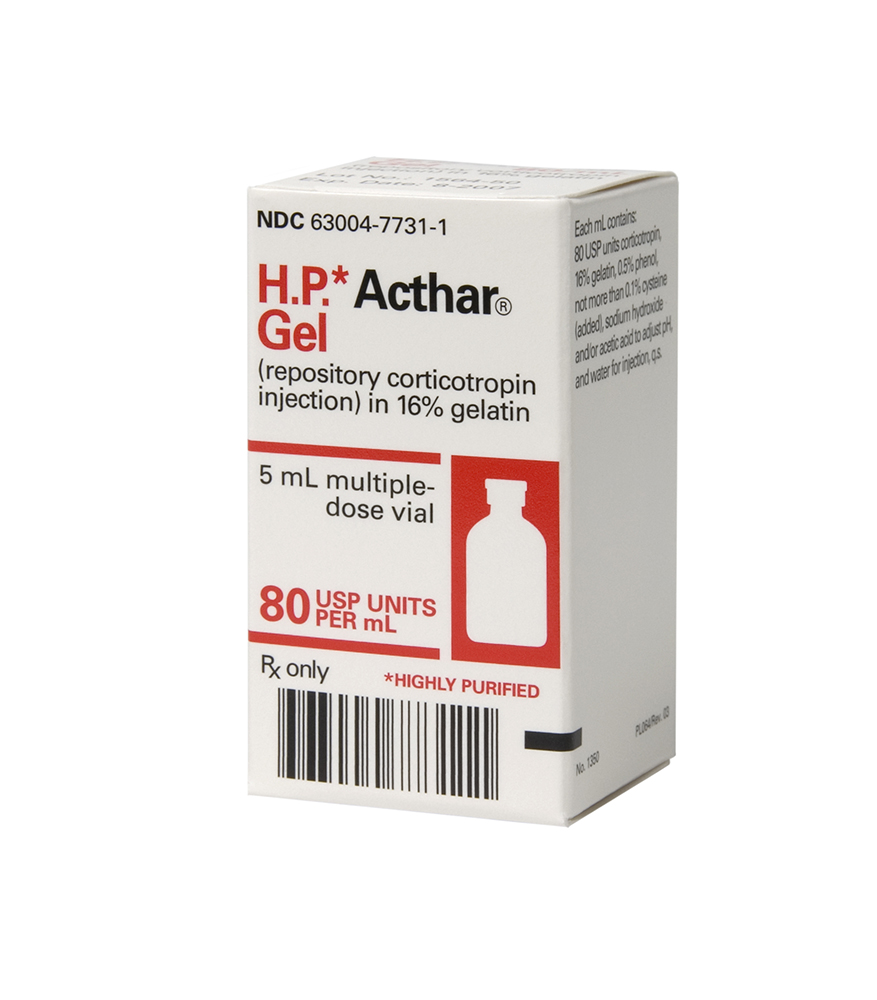
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 5 mL CARTON
H.P.* Acthar®
Gel
(repository corticotropin
injection) in 16% gelatin
5 mL multiple-
dose vial
80 USP UNITS
PER mL
Rx only * HIGHLY PURIFIED
| H.P. ACTHAR
corticotropin injection, solution |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA008372 | 04/29/1952 | |
| Labeler - Questcor Pharmaceuticals, Inc. (625130828) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Cangene bioPharma | 050783398 | manufacture | |