CITRUCEL
-
methylcellulose (4000 cps) powder, for solution
GlaxoSmithKline Consumer Healthcare LP
----------
Drug FactsActive ingredient orange
(in each rounded tablespoon)
Methylcellulose (a non-allergenic fiber) 2g
Active ingredient sugar free
(in each heaping tablespoon)
Methylcellulose (a non-allergenic fiber) 2g
Purpose
Bulk-forming fiber laxative
Uses
- relieves constipation (irregularity)
- helps to restore and maintain regularity
- for constipation associated with other bowel disorders like IBS when recommended by a doctor
- generally produces a bowel movement in 12-72 hours
Warnings
Choking: taking this product without adequate fluid may cause it to swell and block your throat or esophagus and may cause choking. Do not take this product if you have difficulty in swallowing. If you experience chest pain, vomiting, or difficulty swallowing or breathing after taking this product, seek immediate medical attention.
Ask a doctor before use if you have
- a sudden change in bowel habits that persists for two weeks
- abdominal pain, nausea or vomiting
Stop use and ask a doctor if
- constipation lasts more than 7 days
- you have rectal bleeding
These could be signs of a serious condition.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- MIX THIS PRODUCT (CHILD OR ADULT DOSE) WITH AT LEAST 8 OUNCES (A FULL GLASS) OF WATER OR OTHER FLUID. TAKING THIS PRODUCT WITHOUT ENOUGH LIQUID MAY CAUSE CHOKING. SEE CHOKING WARNING
- use product at the first sign of constipation or irregularity
- put one dose in a full glass of cold water
- stir briskly and drink promptly
- drinking another glass of water is helpful
CITRUCEL® Orange
| Age | Dose |
| adults & children 12 years of age and over | start with 1 heaping tablespoon. Increase as needed, 1 heaping tablespoon at a time, up to 3 times per day. |
| children 6 - 11 years of age | start with 2.5 level teaspoons. Increase as needed, 2.5 level teaspoons at a time, up to 3 times per day. |
| children under 6 years of age | consult a physician |
CITRUCEL® Sugar Free
| Age | Dose |
| adults & children 12 years of age and over | start with 1 rounded tablespoon. Increase as needed, 1 rounded tablespoon at a time, up to 3 times per day. |
| children 6 - 11 years of age | start with 2 level teaspoons. Increase as needed, 2 level teaspoons at a time, up to 3 times per day. |
| children under 6 years of age | consult a physician |
Other information
CITRUCEL® Orange
- each heaping tablespoon contains: calcium 80mg and potassium 110mg
- each heaping tablespoon contributes 60 calories from sucrose and maltodextrin
- store below 77°F (25°C)
- protect contents from humidity
- keep tightly closed
CITRUCEL® Sugar Free
- each rounded tablespoon contains: calcium 85mg and potassium 125mg
- each rounded tablespoon contributes 24 calories from maltodextrin
- store below 77°F (25°C)
- protect contents from humidity
- keep tightly closed
- Phenylketonurics: CONTAINS PHENYLALANINE 52mg per adult dose
Inactive ingredients
CITRUCEL® Orange
citric acid, dibasic calcium phosphate, FD&C yellow #6, maltodextrin, orange flavors (natural and artificial), potassium citrate, riboflavin, sucrose, titanium dioxide, tricalcium phosphate
CITRUCEL® Sugar Free
aspartame, dibasic calcium phosphate, FD&C yellow #6, malic acid, maltodextrin, orange flavors (natural and artificial), potassium citrate, riboflavin
Questions or comments?
call toll-free 1-800-897-6081 (English/Spanish) weekdays
CITRUCEL® Orange
Patent No. 4,626,287
4,671,823
4,732,917
CITRUCEL® Sugar Free
Patent No. 4,626,287
4,671,823
Distributed by:
GlaxoSmithKline Consumer Healthcare, L.P.
Moon Township, PA 15108
©2009 GlaxoSmithKline
Citrucel® is a registered trademark of the GlaxoSmithKline group of companies.
Great-Tasting Orange Flavor
CITRUCEL® Orange
CITRUCEL® Sugar Free
with smartfiber®
Citrucel's unique smartfiber® is smart because it is:
- The ONLY fiber that won't ferment to cause excess gas like other fiber products. *
- Clinically proven effective to help restore and maintain regularity.
- Gentle enough for everyday use.
- 100% soluble, non-allergenic and gluten-free.
*Based on laboratory testing. Individual results may vary.
CITRUCEL® Orange
DIRECTIONS FOR USE (NO SCOOP** DOSING):
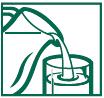
1. Fill glass with at least 8 oz. of cold water.
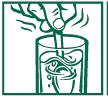
2. Measure heaping tablespoon of product (one dose) and stir product into water. Stir briskly and drink promptly.
**We are as concerned about the environment as you are so we have removed the scoop from our Citrucel powder products. Doing so helps save energy, reduce the amount of plastic produced and improve environmental footprints.
62063XC
5E643 SB F1
CITRUCEL® Sugar Free
DIRECTIONS FOR USE (NO SCOOP** DOSING):
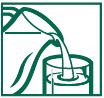
1. Fill glass with at least 8 oz. of cold water.
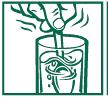
2. Measure rounded tablespoon of product (one dose) and stir product into water. Stir briskly and drink promptly.
**We are as concerned about the environment as you are so we have removed the scoop from our Citrucel powder products. Doing so helps save energy, reduce the amount of plastic produced and improve environmental footprints.
62056XD
5M245 SB F1
Principal Display Panel
Great Taste & Clinically Proven Effective
CITRUCEL®
METHYLCELLULOSE FIBER THERAPY FOR REGULARITY
with smartfiber®
The Only Fiber for Regularity
That Won't Cause Excess Gas*
ORANGE FLAVOR
NET WT 16 OZ (454 GRAMS)

Principal Display Panel
Great Taste & Clinically Proven Effective
CITRUCEL®
METHYLCELLULOSE FIBER THERAPY FOR REGULARITY
with smartfiber®
The Only Fiber for Regularity
That Won't Cause Excess Gas*
sugar free ORANGE FLAVOR
NET WT 32 OZ (907 GRAMS)

| CITRUCEL
methylcellulose powder, for solution |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part334 | 09/16/2010 | |
| CITRUCEL
methylcellulose powder, for solution |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part334 | 09/16/2010 | |
| Labeler - GlaxoSmithKline Consumer Healthcare LP (828924212) |