hydralazine hcl and hydrochlorothiazide (Hydralazine hydrochloride and Hydrochlorothiazide) capsule
[Par Pharmaceutical, Inc.]
WARNING:THIS FIXED-COMBINATION DRUG IS NOT INDICATED FOR INITIAL THERAPY OF HYPERTENSION. HYPERTENSION REQUIRES THERAPY TITRATED TO THE INDIVIDUAL PATIENT. IF THE FIXED COMBINATION REPRESENTS THE DOSAGE SO DETERMINED, ITS USE MAY BE MORE CONVENIENT IN PATIENT MANAGEMENT. THE TREATMENT OF HYPERTENSION IS NOT STATIC BUT MUST BE REEVALUATED AS CONDITIONS IN EACH PATIENT WARRANT.
DESCRIPTION
Hydralazine HCl and hydrochlorothiazide is an antihypertensive-diuretic combination available as capsules for oral administration. Hydralazine HCl and hydrochlorothiazide capsules of 25 mg/25 mg contain 25 mg of Hydralazine hydrochloride USP and 25 mg of hydrochlorothiazide USP; capsules of 50 mg/50 mg contain 50 mg of hydralazine hydrochloride USP and 50 mg of hydrochlorothiazide USP; and capsules of 100 mg/50 mg contain 100 mg of hydralazine hydrochloride USP and 50 mg of hydrochlorothiazide USP.
Each capsule also contains the following inactive ingredients: Corn starch, crospovidone, gelatin, lactose monohydrate, pharmaceutical glaze, sodium starch glycolate, stearic acid, talc, and titanium dioxide. In addition, the 25 mg/25 mg capsule contains ammonium hydroxide, ethylene glycol monoethyl ether, propylene glycol and synthetic black iron oxide; the 50 mg/50 mg capsule contains FD&C Blue #1, FD&C Red #40, FD&C Yellow #6, synthetic red iron oxide and pharmaceutical shellac; and the 100 mg/50 mg capsule contains D&C Red #28, D&C Yellow #10, FD&C Blue #1,FD&C Blue #2, FD&C Red #40, propylene glycol and synthetic black iron oxide.
Hydralazine hydrochloride is 1-hydrazinophthalazine monohydrochloride, and its structural formula is:
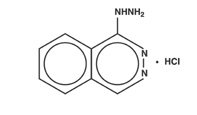
Hydralazine hydrochloride USP is a white to off-white, odorless crystalline powder. It is soluble in water, slightly soluble in alcohol, and very slightly soluble in ether. It melts at about 275°C, with decomposition, and has a molecular weight of 196.64.
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide, and its structural formula is:
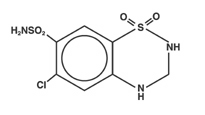
Hydrochlorothiazide USP is a white, or practically white, practically odorless crystalline powder. It is freely soluble in sodium hydroxide solution, inn-butylamine, and in dimethylformamide; sparingly soluble in methanol; slightly soluble in water; and insoluble in ether, in chloroform, and in dilute mineral acids. Its molecular weight is 297.75.
CLINICAL PHARMACOLOGY
Hydralazine
Although the precise mechanism of action of hydralazine is not fully understood, the major effects are on the cardiovascular system. Hydralazine apparently lowers blood pressure by exerting a peripheral vasodilating effect through a direct relaxation of vascular smooth muscle. Hydralazine, by altering cellular calcium metabolism, interferes with the calcium movements within the vascular smooth muscle that are responsible for initiating or maintaining the contractile state.
The peripheral vasodilating effect of hydralazine results in decreased arterial blood pressure (diastolic more than systolic); decreased peripheral vascular resistance; and an increased heart rate, stroke volume, and cardiac output. The preferential dilatation of arterioles, as compared to veins, minimizes postural hypotension and promotes the increase in cardiac output. Hydralazine usually increases renin activity in plasma, presumably as a result of increased secretion of renin by the renal juxtaglomerular cells in response to reflex sympathetic discharge. This increase in renin activity leads to the production of angiotensin II, which then causes stimulation of aldosterone and consequent sodium reabsorption. Hydralazine also maintains or increases renal and cerebral blood flow.
Hydrochlorothiazide
Thiazides affect the renal tubular mechanism of electrolyte reabsorption. At maximal therapeutic dosage, all thiazides are approximately equal in their diuretic potency. Thiazides increase excretion of sodium and chloride in approximately equivalent amounts. Natriuresis causes a secondary loss of potassium.
The mechanism of the antihypertensive effect of thiazides is unknown. Thiazides do not affect normal blood pressure.
Pharmacokinetics
Hydralazine:Hydralazine is rapidly absorbed after oral administration, and peak plasma levels are reached at 1-2 hours. Plasma levels decline with a half-life of 3-7 hours. Binding to human plasma protein is 87%. Plasma levels of hydralazine vary widely among individuals. Hydralazine is subject to polymorphic acetylation; slow acetylators generally have higher plasma levels of hydralazine and require lower doses to maintain control of blood pressure. Hydralazine undergoes extensive hepatic metabolism; it is excreted mainly in the form of metabolites in the urine.
Administration of hydralazine with food results in higher levels of the drug in plasma.
Hydrochlorothiazide: Onset of action of thiazides occurs in 2 hours and the peak effect at about 4 hours. The action persists for approximately 6-12 hours. Hydrochlorothiazide is rapidly absorbed, as indicated by peak concentrations 1-2.5 hours after oral administration. Plasma levels of the drug are proportional to dose; the concentration in whole blood is 1.6-1.8 times higher than in plasma. Thiazides are eliminated rapidly by the kidney. After oral administration of 25 to 100 mg doses, 72-97% of the dose is excreted in the urine, indicating dose-independent absorption. Hydrochlorothiazide is eliminated from plasma in a biphasic fashion with a terminal half-life of 10-17 hours. Plasma protein binding is 67.9%. Plasma clearance is 15.9-30.0 L/hr; volume of distribution is 3.6-7.8 L/kg.
Gastrointestinal absorption of hydrochlorothiazide is enhanced when administered with food. Absorption is decreased in patients with congestive heart failure, and the pharmacokinetics are considerably different in these patients.
INDICATIONS AND USAGE
Hypertension (see boxed WARNING).
CONTRAINDICATIONS
Hydralazine
Hypersensitivity to hydralazine; coronary artery disease; mitral valvular rheumatic heart disease.
Hydrochlorothiazide
Anuria; hypersensitivity to this or other sulfonamide-derived drugs.
WARNINGS
Hydralazine
In a few patients hydralazine may produce a clinical picture simulating systemic lupus erythematosus including glomerulonephritis. In such patients hydralazine should be discontinued unless the benefit-to-risk determination requires continued antihypertensive therapy with this drug. Signs and symptoms usually regress when the drug is discontinued, but residua have been detected many years later. Long-term treatment with steroids may be necessary. (SeePRECAUTIONS, Laboratory Tests.)
Hydrochlorothiazide
Thiazides should be used with caution in patients with severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
Thiazides should be used with caution in patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte imbalance may precipitate hepatic coma.
Thiazides may add to or potentiate the action of other antihypertensive drugs. Potentiation occurs with ganglionic or peripheral adrenergic blocking drugs.
Sensitivity reactions are more likely to occur in patients with a history of allergy or bronchial asthma.
The possibility of exacerbation or activation of systemic lupus erythematosus has been reported.
GENERAL PRECAUTIONS
Hydralazine: Myocardial stimulation produced by hydralazine can cause anginal attacks and ECG changes indicative of myocardial ischemia. The drug has been implicated in the production of myocardial infarction. It must, therefore, be used with caution in patients with suspected coronary artery disease.
The “hyperdynamic” circulation caused by hydralazine may accentuate specific cardiovascular inadequacies. For example, hydralazine may increase pulmonary artery pressure in patients with mitral valvular disease. The drug may reduce the pressor responses to epinephrine. Postural hypotension may result from hydralazine but is less common than with ganglionic blocking agents. It should be used with caution in patients with cerebral vascular accidents.
In hypertensive patients with normal kidneys who are treated with hydralazine, there is evidence of increased renal blood flow and a maintenance of glomerular filtration rate. In some instances where control values were below normal, improved renal function has been noted after administration of hydralazine. However, as with any antihypertensive agent, hydralazine should be used with caution in patients with advanced renal damage.
Peripheral neuritis, evidenced by paresthesia, numbness, and tingling, has been observed. Published evidence suggests that hydralazine has an antipyridoxine effect and that pyridoxine should be added to the regimen if symptoms develop.
Hydrochlorothiazide:All patients receiving thiazide therapy should be observed for clinical signs of fluid or electrolyte imbalance, namely hyponatremia, hypochloremic alkalosis, and hypokalemia (seeLaboratory TestsandDrug/Drug Interactions). Warning signs are dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbance, such as nausea or vomiting.
Hypokalemia may develop, especially in cases of brisk diuresis or severe cirrhosis.
Interference with adequate oral intake of electrolytes will also contribute to hypokalemia. Hypokalemia may be avoided or treated by the use of potassium supplements or foods with a high potassium content.
Any chloride deficit is generally mild and usually does not require specific treatment, except under extraordinary circumstances (as in liver disease or renal disease). Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is water restriction, rather than administration of salt, except in rare instances when the hyponatremia is life-threatening. In cases of actual salt depletion, appropriate replacement is the therapy of choice.
Hyperuricemia may occur or frank gout may be precipitated in certain patients receiving thiazide therapy.
Latent diabetes may become manifest during thiazide administration (see Drug/Drug Interactions).
The antihypertensive effects of the drug may be enhanced in the postsympathectomy patient.
If progressive renal impairment becomes evident, withholding or discontinuing diuretic therapy should be considered.
Calcium excretion is decreased by thiazides. Pathological changes in the parathyroid gland with hypercalcemia and hypophosphatemia have been observed in a few patients on prolonged thiazide therapy. The common complications of hyperparathyroidism, such as renal lithiasis, bone resorption and peptic ulceration, have not been seen.
Thiazide diuretics have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Information for Patients
Patients should be informed of possible side effects and advised to take the medication regularly and continuously as directed.
Laboratory Tests
Hydralazine:Complete blood counts and antinuclear antibody titer determinations are indicated before and periodically during prolonged therapy with hydralazine even though the patient is asymptomatic. These studies are also indicated if the patient develops arthralgia, fever, chest pain, continued malaise, or other unexplained signs or symptoms. A positive antinuclear antibody titer requires that the physician carefully weigh the implications of the test results against the benefits to be derived from antihypertensive therapy with a combination drug containing hydralazine.
Blood dyscrasias, consisting of reduction in hemoglobin and red cell count, leukopenia, agranulocytosis, and purpura, have been reported. If such abnormalities develop, therapy should be discontinued.
Hydrochlorothiazide: Initial and periodic determinations of serum electrolytes to detect possible electrolyte imbalance should be performed at appropriate intervals.
Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids.
Drug Interactions
Hydralazine:MAO inhibitors should be used with caution in patients receiving hydralazine.
When other potent parenteral antihypertensive drugs, such as diazoxide, are used in combination with hydralazine, patients should be continuously observed for several hours for any excessive fall in blood pressure. Profound hypotensive episodes may occur when diazoxide injections and hydralazine are used concomitantly.
Hydrochlorothiazide: Hypokalemia can sensitize or exaggerate the response of the heart to the toxic effects of digitalis (e.g., increased ventricular irritability).
Hypokalemia may develop during concomitant use of steroids or ACTH.
Insulin requirements in diabetic patients may be increased, decreased, or unchanged.
Thiazides may decrease arterial responsiveness to norepinephrine, but not enough to preclude effectiveness of the pressor agent for therapeutic use.
Thiazides may increase the responsiveness to tubocurarine.
Lithium renal clearance is reduced by thiazides, increasing the risk of lithium toxicity.
There have been rare reports in the literature of hemolytic anemia occurring with the concomitant use of hydrochlorothiazide and methyldopa.
Concurrent administration of some nonsteroidal anti-inflammatory agents may reduce the diuretic, natriuretic and antihypertensive effects of thiazide diuretics.
Drug and/or Laboratory Test Interactions
Thiazides may decrease serum levels of protein-bound iodine without signs of thyroid disturbance. Hydralazine HCl and hydrochlorothiazide should be discontinued before tests for parathyroid function are made (seeGeneral, Hydrochlorothiazide, Calcium excretion).
Carcinogenesis and Mutagenesis and Impairment of Fertility
Carcinogenicity, mutagenicity, and fertility studies in animals have not been conducted with hydralazine HCl and hydrochlorothiazide.
Hydralazine:In a lifetime study in Swiss albino mice, there was a statistically significant increase in the incidence of lung tumors (adenomas and adenocarcinomas) of both male and female mice given hydralazine continuously in their drinking water at a dosage of about 250 mg/kg per day (about 80 times the maximum recommended human dose). In a 2-year carcinogenicity study of rats given hydralazine by gavage at doses of 15, 30, and 60 mg/kg per day (approximately 5 to 20 times the recommended human daily dose), microscopic examination of the liver revealed a small, but statistically significant, increase in benign neoplastic nodules in male and female rats from the high-dose group and in female rats from the intermediate-dose group. Benign interstitial cell tumors of the testes were also significantly increased in male rats from the high-dose group. The tumors observed are common in aged rats, and a significantly increased incidence was not observed until 18 months of treatment. Hydralazine was shown to be mutagenic in bacterial systems (Gene Mutation and DNA Repair) and in one of two rat and one rabbit hepatocytein vitroDNA repair studies. Additionalin vivoandin vitrostudies using lymphoma cells, germinal cells, and fibroblasts from mice, bone marrow cells from Chinese hamsters and fibroblasts from human cell lines did not demonstrate any mutagenic potential for hydralazine.
The extent to which these findings indicate a risk to man is uncertain. While long-term clinical observation has not suggested that human cancer is associated with hydralazine use, epidemiologic studies have so far been insufficient to arrive at any conclusions.
Fertility studies in animals have not been conducted with hydralazine.
Hydrochlorothiazide: Two-year feeding studies in mice and rats conducted under the auspices of the National Toxicology Program (NTP) uncovered no evidence of a carcinogenic potential of hydrochlorothiazide in female mice (at doses up to approximately 600 mg/kg/day) or in male and female rats (at doses up to approximately 100 mg/kg/day). The NTP, however, found equivocal evidence for hepatocarcinogenicity in male mice.
Hydrochlorothiazide was not genotoxic inin vitroassays using strains TA 98, TA 100, TA 1535, TA 1537, and TA 1538 ofSalmonella typhimurium(Ames assay) and in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations, or inin vivoassays using mouse germinal cell chromosomes, Chinese hamster bone marrow chromosomes, and theDrosophilasex-linked recessive lethal trait gene. Positive test results were obtained only in thein vitroCHO Sister Chromatid Exchange (clastogenicity) and in the Mouse Lymphoma Cell (mutagenicity) assays, using concentrations of hydrochlorothiazide from 43 to 1300 µg/mL, and in theAspergillus nidulansnondisjunction assay at an unspecified concentration.
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses up to 100 and 4 mg/kg/day, respectively, prior to mating, and throughout gestation.
Pregnancy:Teratogenic Effects.Pregnancy Category C
Animal reproduction studies have not been conducted with hydralazine HCl and hydrochlorothiazide.
Hydralazine:Animal studies indicate that hydralazine is teratogenic in mice at 20-30 times the maximum daily human dose of 200-300 mg and possibly in rabbits at 10-15 times the maximum daily human dose, but that it is nonteratogenic in rats. Teratogenic effects observed were cleft palate and malformations of facial and cranial bones.
Hydrochlorothiazide: Studies in which hydrochlorothiazide was orally administered to pregnant mice and rats during their respective periods of major organogenesis at doses up to 3000 and 1000 mg/kg/day, respectively, provided no evidence of harm to the fetus. There are, however, no adequate and well controlled studies of hydralazine HCl and hydrochlorothiazide in pregnant women. Because animal reproduction studies are not always predictive of human response, this combination drug should be used during pregnancy only if clearly needed.
Nonteratogenic Effects
Hydrochlorothiazide: Thiazides cross the placental barrier and appear in cord blood, and there is a risk of fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in adults.
Nursing Mothers
It is not known whether hydralazine is excreted in human milk. Thiazides are excreted in human milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness of the combination drug in children have not been established.
ADVERSE REACTIONS
Adverse reactions are usually reversible upon reduction of dosage or discontinuation of hydralazine HCl and hydrochlorothiazide. Whenever adverse reactions are moderate or severe, it may be necessary to discontinue the drug.
Hydralazine: The following adverse reactions have been observed, but there has not been enough systematic collection of data to support an estimate of their frequency.
Common
Headache, anorexia, nausea, vomiting, diarrhea, palpitations, tachycardia, angina pectoris.
Less Frequent
Digestive:Constipation, paralytic ileus.
Cardiovascular: Hypotension, paradoxical pressor response, edema.
Respiratory:Dyspnea.
Neurologic: Peripheral neuritis, evidenced by paresthesia, numbness, and tingling; dizziness; tremors; muscle cramps; psychotic reactions characterized by depression, disorientation, or anxiety.
Genitourinary:Difficulty in urination.
Hematologic:Blood dyscrasias, consisting of reduction in hemoglobin and red cell count, leukopenia, agranulocytosis, purpura; lymphadenopathy; splenomegaly.
Hypersensitive Reactions: Rash, urticaria, pruritus, fever, chills, arthralgia, eosinophilia, and rarely, hepatitis.
Other:Nasal congestion, flushing, lacrimation, conjunctivitis.
Hydrochlorothiazide:The following adverse reactions have been observed but there has not been enough systematic collection of data to support an estimate of their frequency. Consequently the reactions are categorized by organ systems and are listed in decreasing order of severity and not frequency.
Digestive:Pancreatitis, jaundice (intrahepatic cholestatic), sialadenitis, vomiting, diarrhea, cramping, nausea, gastric irritation, constipation, anorexia.
Cardiovascular: Orthostatic hypotension (may be potentiated by alcohol, barbiturates, or narcotics).
Neurologic: Vertigo, dizziness, transient blurred vision, headache, paresthesia, xanthopsia, weakness, restlessness.
Musculoskeletal: Muscle spasm.
Hematologic:Aplastic anemia, agranulocytosis, leukopenia, thrombocytopenia.
Metabolic: Hyperglycemia, glycosuria, hyperuricemia.
Hypersensitive Reactions: Necrotizing angitis, Stevens-Johnson syndrome, respiratory distress including pneumonitis and pulmonary edema, purpura, urticaria, rash, photosensitivity.
OVERDOSAGE
Acute Toxicity
Oral LD50’s in rats (mg/kg): hydralazine, 173 and 187; hydrochlorothiazide, 2750.
Signs and Symptoms
Hydralazine: Signs and symptoms of overdosage include hypotension, tachycardia, headache, and generalized skin flushing.
Complications can include myocardial ischemia and subsequent myocardial infarction, cardiac arrhythmia and profound shock.
Hydrochlorothiazide: The most prominent feature of poisoning is acute loss of fluid and electrolytes.
Cardiovascular: Tachycardia, hypotension, shock.
Neuromuscular:Weakness, confusion, dizziness, cramps of the calf muscles, paresthesia, fatigue, impairment of consciousness.
Digestive: Nausea, vomiting, thirst.
Renal:Polyuria, oliguria or anuria (due to hemoconcentration).
Laboratory Findings: Hypokalemia, hyponatremia, hypochloremia, alkalosis; increased BUN (especially in patients with renal insufficiency).
Combined Poisoning: Signs and symptoms may be aggravated or modified by concomitant intake of antihypertensive medication, barbiturates, curare, digitalis (hypokalemia), corticosteroids, narcotics, or alcohol.
Treatment
There is no specific antidote.
The gastric contents should be evacuated, taking adequate precautions against aspiration and for protection of the airway. An activated charcoal slurry may be instilled if conditions permit. Dialysis may not be effective for elimination of hydralazine HCl and hydrochlorothiazide because of its plasma protein binding (seeCLINICAL PHARMACOLOGY).
These manipulations may have to be omitted or carried out after cardiovascular status has been stabilized, since they might precipitate cardiac arrhythmias or increase the depth of shock.
Support of the cardiovascular system is of primary importance in suspected hydralazine overdosage. Shock should be treated with plasma expanders. The patient’s legs should be kept raised and lost fluid and electrolytes (potassium, sodium) should be replaced. If possible, vasopressors should not be given, but if a vasopressor is required, care should be taken not to precipitate or aggravate cardiac arrhythmia. Tachycardia responds to beta blockers. Digitalization may be necessary, and renal function should be monitored and supported as required.
DOSAGE AND ADMINISTRATION
Dosage should be determined by individual titration (see boxedWARNING).
The usual dosage is one hydralazine HCl and hydrochlorothiazide capsule twice daily, the strength depending upon individual requirement following titration. For maintenance, the dosage should be adjusted to the lowest effective level.
When necessary, other antihypertensive agents such as sympathetic inhibitors may be added gradually in reduced dosages, and the effects should be watched carefully.
HOW SUPPLIED
Hydralazine HCl and hydrochlorothiazide are supplied as two piece hard gelatin capsules, in three dosage strengths:
25 mg hydralazine hydrochloride and 25 mg hydrochlorothiazide capsules are white and imprinted “Par 143”, and are available in bottles of 100 (NDC #49884-143-01), 500 (NDC #49884-143-05) and 1000 (NDC #49884-143-10).
50 mg hydralazine hydrochloride and 50 mg hydrochlorothiazide capsules are white/black and imprinted “Par 144”, and are available in bottles of 100 (NDC #49884-144-01), 500 (NDC #49884-144-05) and 1000 (NDC #49884-144-10).
100 mg hydralazine hydrochloride and 50 mg hydrochlorothiazide capsules are powder blue/light blue and imprinted “Par 145”, and are available in bottles of 100 (NDC #49884-145-01), 500 (NDC #49884-145-05) and 1000 (NDC #49884-145-10).
Store at controlled room temperature 15°-30°C (59°-86°F).
Dispense in a tight, light-resistant container as defined in the USP.
Manufactured by:
PAR PHARMACEUTICAL, INC.
Spring Valley, NY 10977
| Rev: 03/05 | OS143-01-1-15 | |
| Hydralazine HCl and Hydrochlorothiazide (Hydralazine hydrochloride and Hydrochlorothiazide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydralazine HCl and Hydrochlorothiazide (Hydralazine hydrochloride and Hydrochlorothiazide) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydralazine HCl and Hydrochlorothiazide (Hydralazine hydrochloride and Hydrochlorothiazide) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Revised: 03/2007