ISAGEL
-
alcohol gel
Coloplast Manufacturing US, LLC
----------
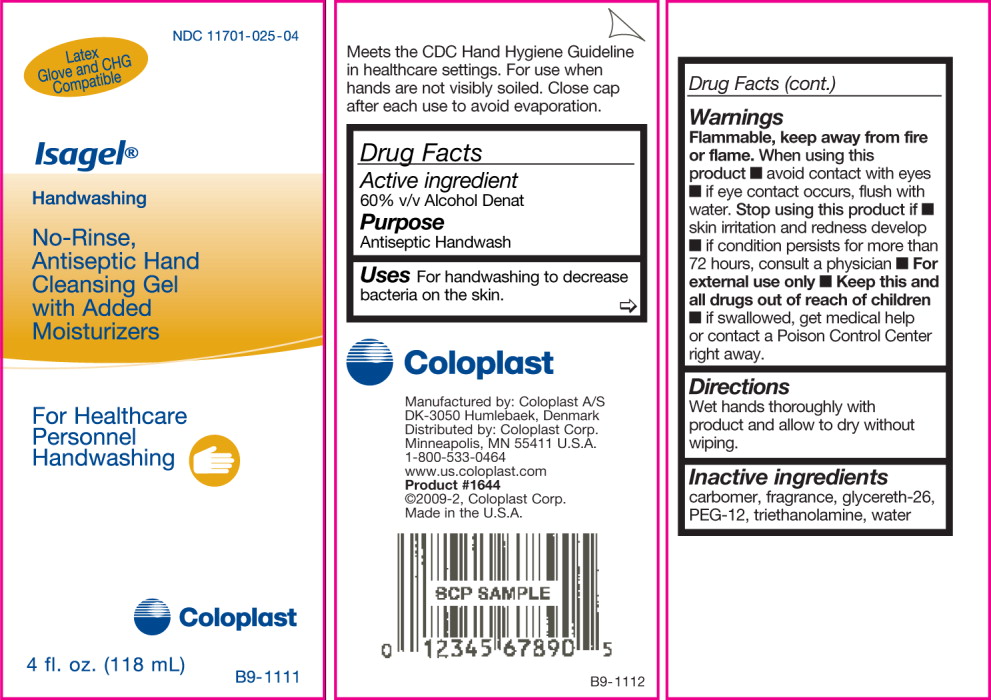
Isagel®Handwashing
No-Rinse, Antiseptic Hand Cleansing Gel with Added Moisturizers
For Healthcare Personnel Handwashing
Drug Facts
Active ingredient
60% v/v Alcohol Denat
Purpose
Antiseptic Healthcare
Personnel Handwash
Uses
For handwashing to decrease bacteria on the skin.
Warnings
Flammable, keep away from fire or flame.
When using this product
- avoid contact with eyes
- if eye contact occurs, flush with water.
Stop using this product
- if skin irritation and redness develop.
- If condition persists for more than 72 hours, consult a physician
For external use only
Keep this and all drugs out of reach of children. If swallowed,get medical help or contact a Poison Control Center right away.
Directions
Wet hands thoroughly with product and allow to dry without wiping.
Inactive ingredients
carbomer, fragrance, glycereth-26, PEG-12, triethanolamine, water
Meets the CDC Hand Hygiene Guideline in healthcare settings. For use when hands are not visibly soiled.
Manufactured by: Coloplast A/S
DK-3050 Humlebaek, Denmark
Distributed by: Coloplast Corp.
Minneapolis, MN 55411 U.S.A.
1-800-533-0464 www.us.coloplast.com
Product #1645 ©2009-2, Coloplast Corp.
Made in the U.S.A.
DSP-MN-38
PRINCIPAL DISPLAY PANEL - 21 fl. oz. (621 mL)
NDC 11701-025-26
Latex Clove and CHG Compatible
Isagel®
Handwashing
No-Rinse, Antiseptic Hand Cleansing Gel with Added Moisturizers
For Healthcare Personnel Handwashing
Coloplast
21 fl. oz. (621 mL)
| ISAGEL
alcohol gel |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333 | 06/15/2009 | |
| Labeler - Coloplast Manufacturing US, LLC (110326675) |
| Registrant - Coloplast Corp (847436391) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Coloplast Manufacturing US, LLC | 110326675 | MANUFACTURE | |