ASPIRIN LOW DOSE ENTERIC COATED
-
aspirin tablet, coated
Time-Cap Labs, Inc
----------
DRUG FACTSDrug Facts
Active ingredient (in each tablet) Purpose
Aspirin 81 mg (NSAID*). . . . . . . . . . . . . . . . . . .Pain reliever
*nonsteroidal anti-inflammatory drug
Ask a doctor before use if • stomach bleeding warning
applies to you • you have a history of stomach
problems, such as heartburn • you have high blood
pressure, heart disease, liver cirrhosis, or kidney disease
• you are taking a diuretic • you have asthma • you
have not been drinking fluids • you have lost a lot of
fluid due to vomiting or diarrhea
Ask a doctor or pharmacist before use if you are
• taking a prescription drug for diabetes, gout, or arthritis
• under a doctor’s care for any serious condition
• taking any other drug
the following signs of stomach bleeding: • feel faint
• have bloody or black stools • vomit blood
• have stomach pain that does not get better
• pain gets worse or lasts more than 10 days
• fever gets worse or lasts more than 3 days
• redness or swelling is present in the painful area
• any new symptoms appear
• ringing in the ears or a loss of hearing occurs
not use • if
you are allergic to aspirin or any other
pain reliever/fever reducer
If pregnant or breast-feeding, ask a health professional before
use. It is especially important not to use aspirin during the last
3 months of pregnancy unless definitely directed to do so by a
doctor because it may cause problems in the unborn child or
complications during delivery.
Keep out of reach of children. In case of overdose, get medical
help or contact a Poison Control Center right away.
Ask a doctor or pharmacist before use if you are
• taking a prescription drug for diabetes, gout, or arthritis
• under a doctor’s care for any serious condition
• taking any other drug
Uses • temporarily relieves minor aches and pains
• for other uses, see your doctor, but do not use for more
than 10 days without consulting your doctor because
serious side effects may occur
NDC 49483-387-10LOW DOSE ASPIRIN
REGIMEN ENTERIC safety Coated 1000 ENTERIC COATED TABLETS SEE NEW 81MG WARNINGS INFORMATION
Pain Reliever(NSAID) New Improved Formula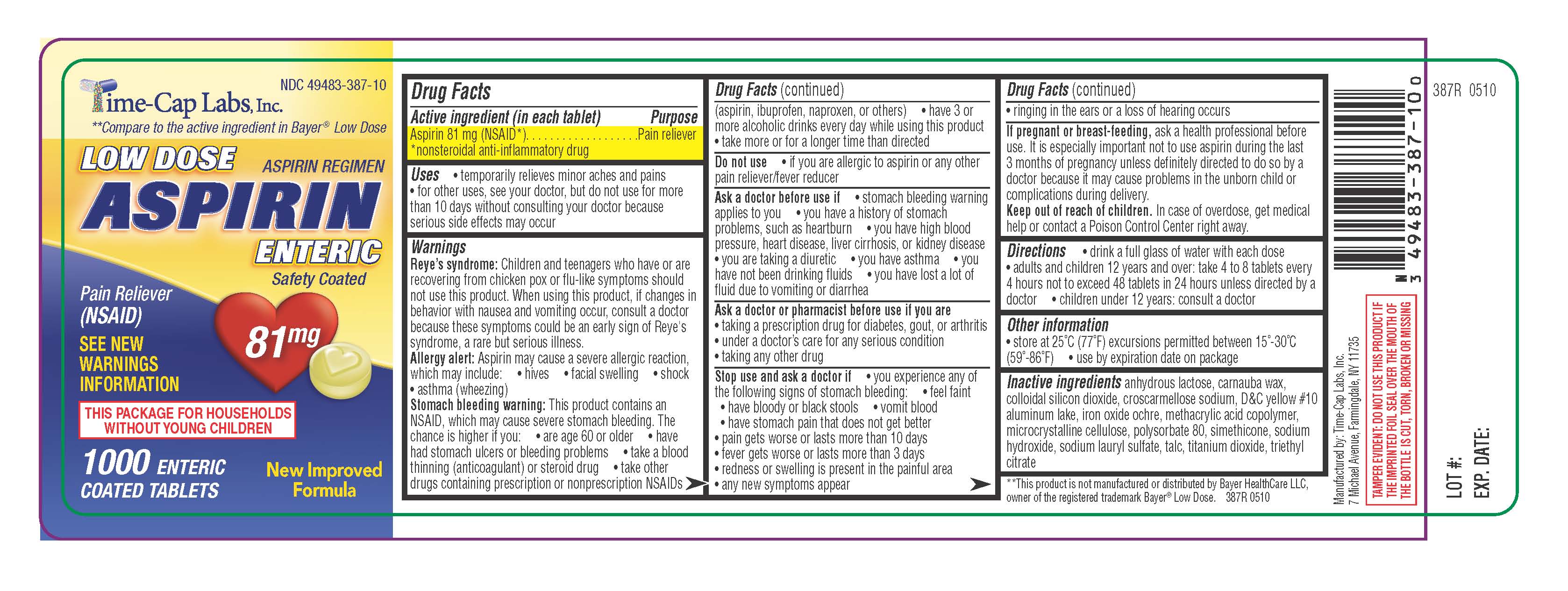
| ASPIRIN
LOW DOSE ENTERIC COATED
aspirin tablet, coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part343 | 06/28/2010 | |
| Labeler - Time-Cap Labs, Inc (037052099) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Time-Cap Labs, Inc | 037052099 | manufacture | |