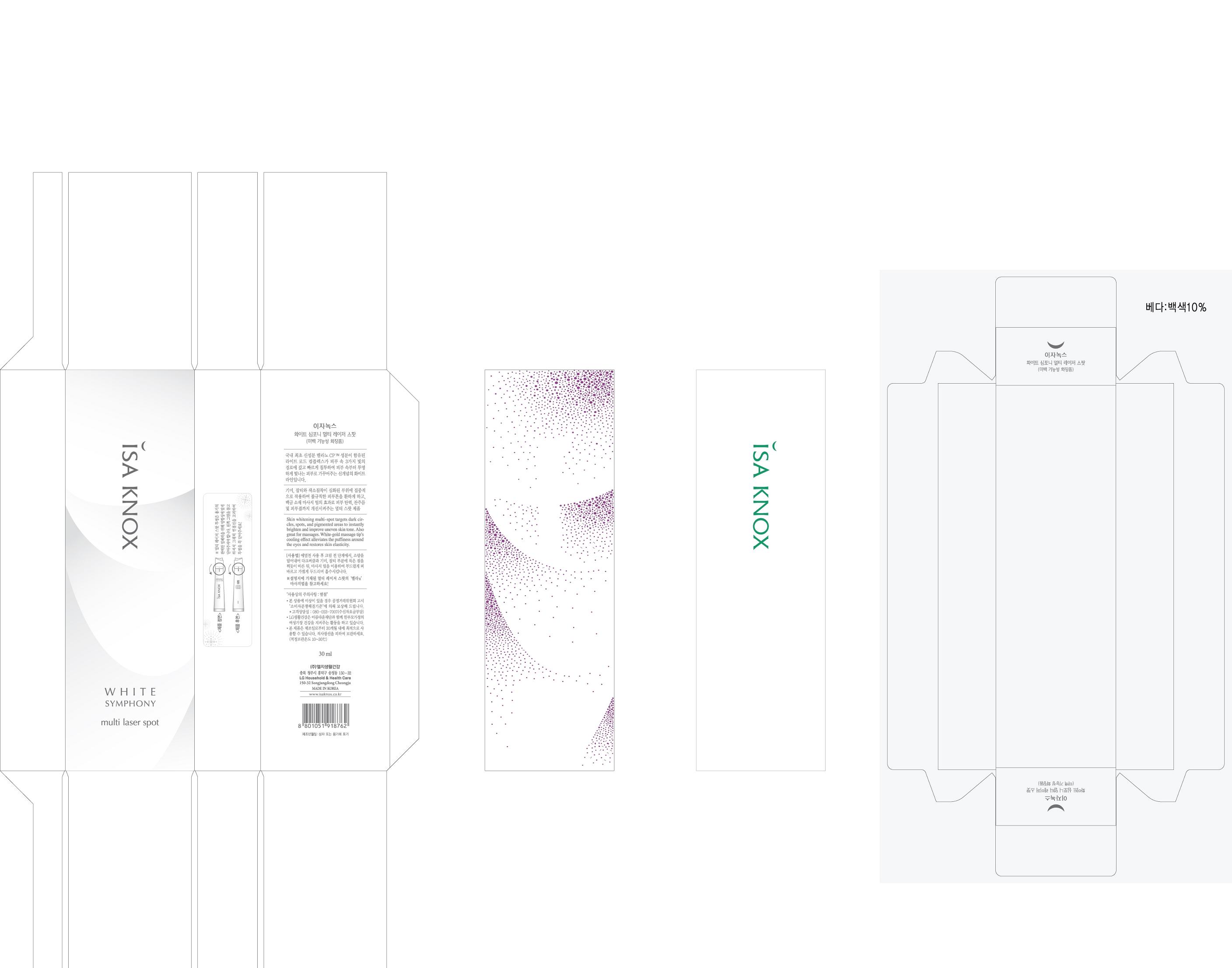
ISAKNOX WHITE SYMPHONY MULTI LASER SPOT
-
dihydroxymethoxychalcone cream
LG Household and Healthcare, Inc.
----------
Drug FactsActive Ingredients
DIHYDROXYMETHOXYCHALCONE 0.05 ml/100ml
WARNING AND PRECAUTIONS
For external use only.
KEEP OUT OF REACH OF CHILDREN
Keep out of eyes. Rinse with water to remove.
WHEN USING
Keep out of eyes. Rinse with water to remove.
ISAKNOX
WHITE SYMPHONY
MULTI LASER SPOT
| ISAKNOX WHITE SYMPHONY MULTI LASER SPOT
dihydroxymethoxychalcone cream |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 07/01/2010 | ||
| Labeler - LG Household and Healthcare, Inc. (688276187) |
| Registrant - LG Household and Healthcare, Inc. (688276187) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LG Household and Healthcare, Inc. | 688276187 | manufacture | |