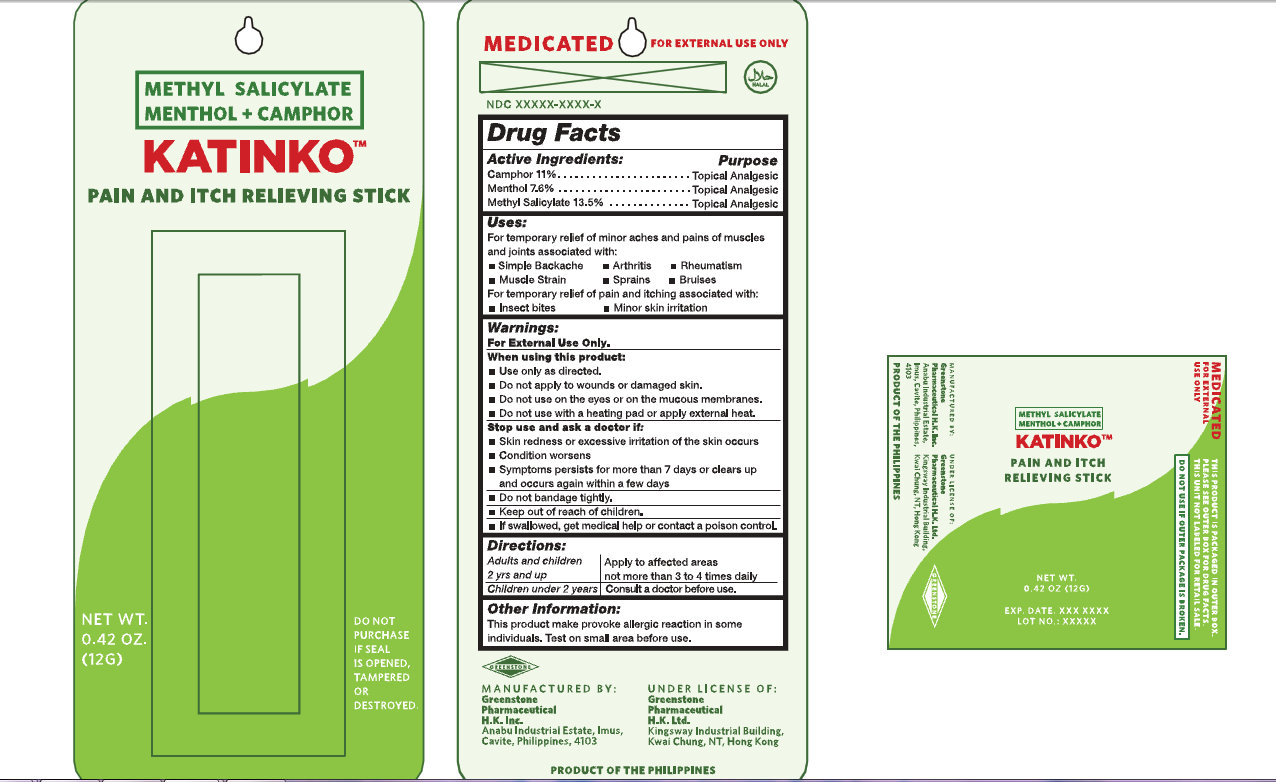
KATINKO PAIN AND ITCH RELIEVING
-
camphor (synthetic),
menthol and
methyl salicylate stick
Greenstone Pharmaceutical H.K. Inc.
----------
Katinko Pain And Itch Relieving StickActive Ingredients: Purpose
Camphor 11 percent Topical Analgesic
Menthol 7.6 percent Topical Analgesic
Methyl Salicylate 13.5 percent Topical Analgesic
Uses:
For temporary relief of minor aches and pains of muscles and joints associated with:
Simple Backache Arthritis Rheumatism Muscle Strain Sprains Bruises
For temporary relief of pain and itching associated with:
Insect Bites Minor skin irritation
Warnings
For External Use Only.
When using this product:
Use only as directed.
Do not apply to wounds or damaged skin.
Do not use on the eyes or on the mucous membranes.
Do not use with a heating pad or apply external heat.
Stop use and ask a doctor if:
Skin redness or excessive irritation of the skin occurs
Condition worsens
Symptoms persists for more than 7 days or clears up and occurs again within a few days
Do not bandage tightly
Keep out of reach of children
If swallowed, get medical help or contact a poison control
Directions:
Adults and children 2 yrs and up Apply to affected areas not more than 3 to 4 times daily
Children under 2 years Consult a doctor before use
Other Information
This product make provoke allergic reaction in some individuals. Test on small area before use.
MANUFACTURED BY
Greenstone Pharmaceutical H.K. Inc.
Anabu Industrial Estate, Imus, Cavite, Philippines, 4103
UNDER LICENSE OF:
Greenstone Pharmaceutical H.K. Ltd
Kingsway Industrial Building, Kwai Chung, NT, Hong Kong
METHYL SALICYLATE
MENTHOL
CAMPHOR
KATINKO
PAIN AND ITCH RELIEVING STICK
NET WT
0.42 OZ
(12G)
DO NOT PURCHASE IF SEAL IS OPENED, TAMPERED OR DESTROYED.
| KATINKO PAIN AND ITCH RELIEVING
camphor (synthetic), menthol, methyl salicylate stick |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part348 | 05/24/2010 | |
| Labeler - Greenstone Pharmaceutical H.K. Inc. (719794307) |
| Registrant - Greenstone Pharmaceutical H.K. Inc. (719794307) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Greenstone Pharmaceutical H.K. Inc. | 719794307 | manufacture | |