MEDIQUE DECOREL FORTE PLUS
-
acetaminophen,
dextromethorphan hydrobromide,
guaifenesin and
phenylephrine hydrochloride tablet, film coated
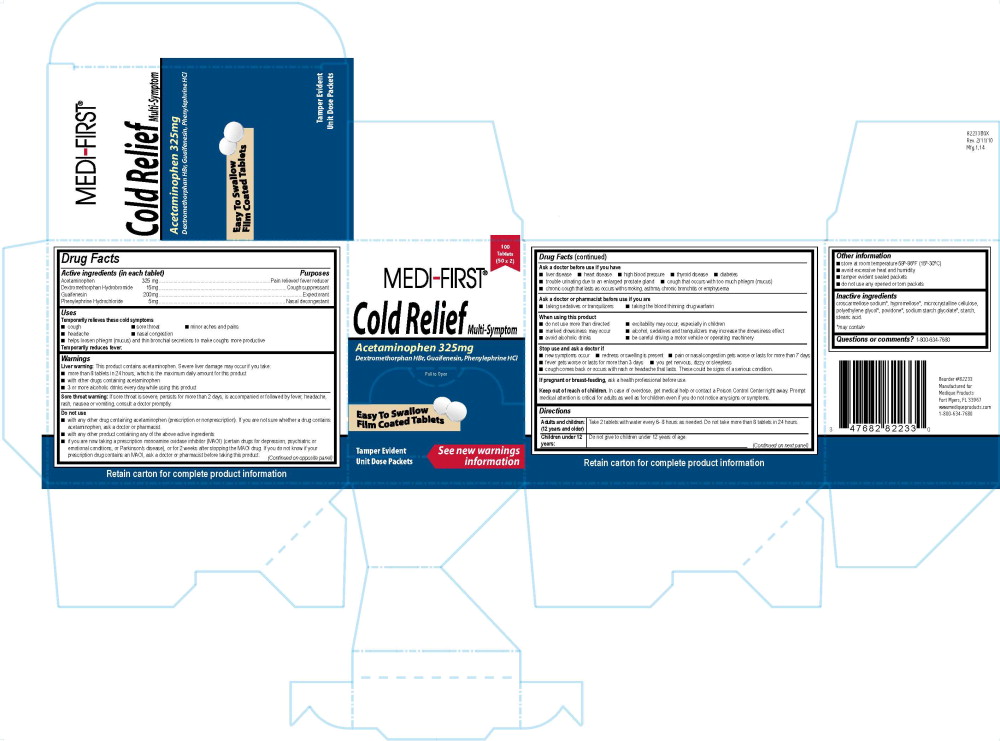
MEDI-FIRST COLD RELIEF
-
acetaminophen,
dextromethorphan hydrobromide,
guaifenesin and
phenylephrine hydrochloride tablet, film coated
MEDI-FIRST PLUS COLD RELIEF
-
acetaminophen,
dextromethorphan hydrobromide,
guaifenesin and
phenylephrine hydrochloride tablet, film coated
Unifirst First Aid Corporation
----------
425R Decorel Forte Plus UniFirst 47682 Medique, MF/MFPDrug Facts
Active ingredients (in each tablet)
Acetaminophen 325 mg
Dextromethorphan Hydrobromide 15mg
Guaifenesin 200mg
Phenylephrine Hydrochloride 5mg
Purposes
Pain reliever/ fever reducer
Cough suppressant
Expectorant
Nasal decongestant
Uses
Temporarily relieves these cold symptoms
- cough
- sore throat
- minor aches and pains
- headache
- nasal congestion
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
Temporarily reduces fever.
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 8 tablets in 24 hours, which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with any other product containing any of the above active ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- cough that occurs with too much phlegm (mucus)
- chronic cough that lasts as occurs with smoking, asthma, chronic bronchitis or emphysema
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- do not use more than directed
- excitability may occur, especially in children
- marked drowsiness may occur
- alcohol, sedatives and tranquilizers may increase the drowsiness effect
- avoid alcoholic drinks
- be careful driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- new symptoms occur
- redness or swelling is present
- pain or nasal congestion gets worse or lasts for more than 7 days
- fever gets worse or lasts for more than 3 days
- you get nervous, dizzy or sleepless
- cough comes back or occurs with rash or headache that lasts. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
Adults and children: (12 years and older)
Take 2 tablets with water every 6- 8 hours as needed. Do not take more than 8 tablets in 24 hours.
Children under 12 years:
Do not give to children under 12 years of age.
Other information
- store at room temperature 59°-86°F (15°-30°C)
- avoid excessive heat and humidity
- tamper evident sealed packets
- do not use any opened or torn packets
Inactive ingredients
croscarmellose sodium*, hypromellose*, microcrystalline cellulose, polyethylene glycol*, povidone*, sodium starch glycolate*, starch, stearic acid.
*may contain
Questions or comments? 1-800-634-7680
425R Medique Decorel Forte Plus Label
Collect Medi-Bucks
See inside flap for more details
Medique®
Decorel Forte Plus
Acetaminophen/Acetamonofeno 325mg
Dextromethorphan HBr/Hidrobromuro de dextrometorfano 15mg
Guaifenesin/Guaifenesina 200mg
Phenylephrine HCl/Hidrocloruro de fenilefrina 5mg
Easy To Swallow
Film Coated Tablets
Facil de Tragar Tabletas con Cubierta Pelicular
Pull to Open
TiraParaAbrir
See new warnings information
500 Tablets
(250 x 2)
Severe Cold and Cough Relief
Alivio para severos resfriados y para la tos
Tamper Evident Unit Dose Packets
Empaquetado con Sellado Evidente en Dosis Untarias
425R MF Cold Relief Label
100 Tablets
(50 x 2)
Medi-First®
Cold Relief Multi-Symptom
Acetaminophen 325mg
Dextromethorphan HBr, Guaifenesin, Phenylephrine HCl
Pull to Open
Easy To Swallow
Film Coated Tablets
See new warnings information
Tamper Evident Unit Dose Packets
425R MFP Cold Relief Label
250 Tablets
(125 x 2's)
Medi-First® Plus
Cold Relief Multi-Symptom
See new warnings information
Pull to Open
Acetaminophen 325mg
Dextromethorphan HBr, Guaifenesin, Phenylephrine HCl
Easy To Swallow
Film Coated Tablets
| MEDIQUE DECOREL FORTE PLUS
acetaminophen, dextromethorphan hydrobromide, guaifenesin, phenylephrine hydrochloride tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 12/30/2008 | |
| MEDI-FIRST COLD RELIEF
acetaminophen, dextromethorphan hydrobromide, guaifenesin, phenylephrine hydrochloride tablet, film coated |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 12/30/2008 | |
| MEDI-FIRST PLUS COLD RELIEF
acetaminophen, dextromethorphan hydrobromide, guaifenesin, phenylephrine hydrochloride tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 12/30/2008 | |
| Labeler - Unifirst First Aid Corporation (832947092) |