VICKS VAPOR INHALER
-
levmetamfetamine inhalant
Procter & Gamble Manufacturing Company
----------
Vicks®Vapor Inhaler
Drug Facts
Active ingredient (per inhaler)
Levmetamfetamine 50 mg
Purpose
Nasal decongestant
Uses
temporarily relieves nasal congestion due to:
- a cold
- hay fever or other upper respiratory allergies
Warnings
When using this product
- do not exceed recommended dosage
- temporary burning, stinging, sneezing, or increased nasal discharge may occur
- use of this container by more than one person may spread infection
- do not use for more than 7 days
- use only as directed
- frequent or prolonged use may cause nasal congestion to recur or worsen
Stop use and ask a doctor if symptoms persist
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- the product delivers into each 800 ml of air 0.04 to 0.150 mg of levmetamfetamine
- do not use more often than every 2 hours
| adults and children 12 years and over | 2 inhalations in each nostril |
| children 6 to under 12 years (with adult supervision) | 1 inhalation in each nostril |
| children 2 to under 6 years | ask a doctor |
| children under 2 years | do not use |
Other information
- store at room temperature
- keep inhaler tightly closed
- this inhaler is effective for a minimum of 3 months after first use
Inactive ingredients
bornyl acetate, camphor, lavender oil, menthol, methyl salicylate
Questions?
1-800-873-8276
Dist. by Procter & Gamble, Cincinnati OH 45202.
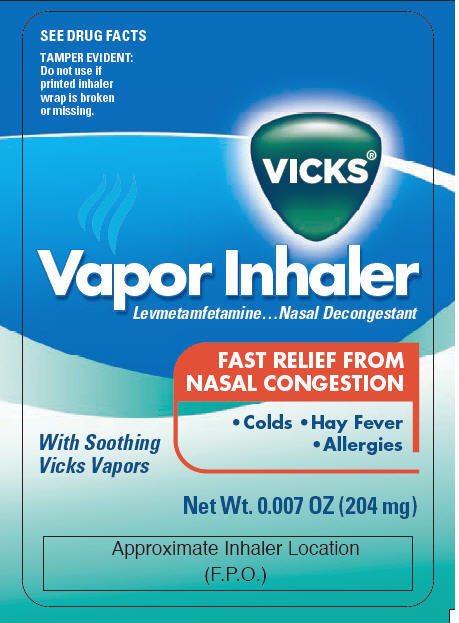
PRINCIPAL DISPLAY PANEL - 204 mg Bottle Carton
SEE DRUG FACTS
TAMPER EVIDENT:
Do not use if
printed inhaler
wrap is broken
or missing.
VICKS®
Vapor Inhaler
Levmetamfetamine…Nasal Decongestant
FAST RELIEF FROM
NASAL CONGESTION
• Colds • Hay Fever
• Allergies
With Soothing
Vicks Vapors
Net Wt. 0.007 OZ (204 mg)
Approximate Inhaler Location
(F.P.O.)
 |  |
| VICKS
VAPOR INHALER
levmetamfetamine inhalant |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 02/28/1995 | 07/09/2009 |
| Labeler - Procter & Gamble Manufacturing Company (004238200) |