ambien cr (zolpidem tartrate) tablet, coated
[sanofi-aventis U.S. LLC]
DESCRIPTION
Ambien CR contains zolpidem tartrate, a non-benzodiazepine hypnotic of the imidazopyridine class. Ambien CR (zolpidem tartrate extended-release tablets) is available in 6.25-mg and 12.5-mg strength tablets for oral administration.
Chemically, zolpidem tartrate is N,N,6-trimethyl-2-p-tolylimidazo[1,2-a] pyridine-3-acetamide L-(+)-tartrate (2:1). It has the following structure:
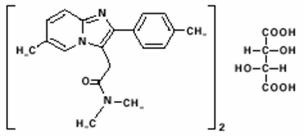
Zolpidem tartrate is a white to off-white crystalline powder that is sparingly soluble in water, alcohol, and propylene glycol. It has a molecular weight of 764.88.
Ambien CR consists of a coated two-layer tablet: one layer that releases its drug content immediately and another layer that allows a slower release of additional drug content. The 6.25-mg Ambien CR tablet contains the following inactive ingredients: colloidal silicon dioxide, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, potassium bitartrate, red ferric oxide, sodium starch glycolate, and titanium dioxide. The 12.5-mg Ambien CR tablet contains the following inactive ingredients: colloidal silicon dioxide, FD&C Blue #2, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, potassium bitartrate, sodium starch glycolate, titanium dioxide, and yellow ferric oxide.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Subunit modulation of the GABAA receptor chloride channel macromolecular complex is hypothesized to be responsible for sedative, anticonvulsant, anxiolytic, and myorelaxant drug properties. The major modulatory site of the GABAA receptor complex is located on its alpha (α) subunit and is referred to as the benzodiazepine (BZ) receptor.
Zolpidem, the active moiety of zolpidem tartrate, is a hypnotic agent with a chemical structure unrelated to benzodiazepines, barbiturates, pyrrolopyrazines, pyrazolopyrimidines, or other drugs with known hypnotic properties. In contrast to the benzodiazepines, which nonselectively bind to and activate all BZ receptor subtypes, zolpidem in vitro binds the BZ1 receptor preferentially with a high affinity ratio of the alpha1/alpha5 subunits. The BZ1 receptor is found primarily on the Lamina IV of the sensorimotor cortical regions, substantia nigra (pars reticulata), cerebellum molecular layer, olfactory bulb, ventral thalamic complex, pons, inferior colliculus, and globus pallidus. This selective binding of zolpidem on the BZ1 receptor is not absolute, but it may explain the relative absence of myorelaxant and anticonvulsant effects in animal studies as well as the preservation of deep sleep (stages 3 and 4) in human studies of zolpidem at hypnotic doses.
Pharmacokinetics
Ambien CR exhibits biphasic absorption characteristics, which results in rapid initial absorption from the gastrointestinal tract similar to zolpidem tartrate immediate-release, then provides extended plasma concentrations beyond three hours after administration. A study in 24 healthy male subjects was conducted to compare mean zolpidem plasma concentration-time profiles obtained after single oral administration of Ambien CR (12.5 mg) and of an immediate-release formulation of zolpidem tartrate (10 mg). The terminal elimination half-life observed with Ambien CR (12.5 mg) was similar to that obtained with immediate-release zolpidem tartrate (10 mg). The mean plasma concentration time profiles for Ambien CR (12.5 mg) and for zolpidem tartrate (10 mg) are shown below:
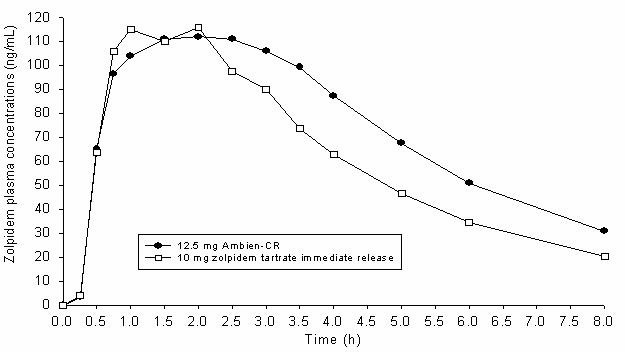
In adult and elderly patients treated with Ambien CR, there was no evidence of accumulation after repeated once-daily dosing for up to two weeks.
Absorption
Following administration of Ambien CR, administered as a single 12.5-mg dose in healthy male adult subjects, the mean peak concentration (Cmax) of zolpidem was 134 ng/mL (range: 68.9 to 197 ng/ml) occurring at a median time (Tmax) of 1.5 hours. The mean AUC of zolpidem was 740 ng∙hr/mL (range: 295 to 1359 ng∙hr/mL).
A food-effect study in 45 healthy volunteers compared the pharmacokinetics of Ambien CR 12.5 mg when administered while fasting or within 30 minutes after a meal. Results demonstrated that with food, mean AUC and Cmax were decreased by 23% and 30%, respectively, while median Tmax was increased from 2 hours to 4 hours. The half-life was not changed. These results suggest that, for faster sleep onset, Ambien CR should not be administered with or immediately after a meal.
Distribution
Total protein binding was found to be 92.5 ± 0.1% and remained constant, independent of concentration between 40 and 790 ng/mL.
Metabolism
Zolpidem is converted to inactive metabolites that are eliminated primarily by renal excretion.
Elimination
Ambien CR administered as a single 12.5 mg dose in healthy male adult subjects, the mean zolpidem elimination half-life was 2.8 hours (range: 1.62 to 4.05 hr).
Special Populations
Elderly
In 24 elderly (≥65 years) healthy subjects administered a single 6.25-mg dose of Ambien CR, the mean peak concentration (Cmax) of zolpidem was 70.6 (range: 35.0 to 161) ng/mL occurring at a median time (Tmax) of 2.0 hours. The mean AUC of zolpidem was 413 ng∙hr/mL (range: 124 to 1190 ng∙hr/mL) and the mean elimination half-life was 2.9 hours (range: 1.59 to 5.50 hours).
Hepatic Impairment
Ambien CR was not studied in patients with hepatic impairment. The pharmacokinetics of an immediate-release formulation of zolpidem tartrate in eight patients with chronic hepatic insufficiency were compared to results in healthy subjects. Following a single 20-mg oral zolpidem tartrate dose, mean Cmax and AUC were found to be two times (250 vs 499 ng/mL) and five times (788 vs 4,203 ng∙hr/mL) higher, respectively, in hepatically compromised patients. Tmax did not change. The mean half-life in cirrhotic patients of 9.9 hr (range: 4.1 to 25.8 hr) was greater than that observed in normals of 2.2 hr (range: 1.6 to 2.4 hr). Dosing should be modified accordingly in patients with hepatic insufficiency (see Precautions and Dosage and Administration).
Renal Impairment
Ambien CR was not studied in patients with renal impairment. The pharmacokinetics of an immediate-release formulation of zolpidem tartrate were studied in 11 patients with end-stage renal failure (mean ClCr = 6.5 ± 1.5 mL/min) undergoing hemodialysis three times a week, who were dosed with zolpidem tartrate 10 mg orally each day for 14 or 21 days. No statistically significant differences were observed for Cmax, Tmax, half-life, and AUC between the first and last day of drug administration when baseline concentration adjustments were made. On day 1, Cmax was 172 ± 29 ng/mL (range: 46 to 344 ng/mL). After repeated dosing for 14 or 21 days, Cmax was 203 ± 32 ng/mL (range: 28 to 316 ng/mL). On day 1, Tmax was 1.7 ± 0.3 hr (range: 0.5 to 3.0 hr); after repeated dosing Tmax was 0.8 ± 0.2 hr (range: 0.5 to 2.0 hr). This variation is accounted for by noting that last-day serum sampling began 10 hours after the previous dose, rather than after 24 hours. This resulted in residual drug concentration and a shorter period to reach maximal serum concentration. On day 1, T1/2 was 2.4 ± 0.4 hr (range: 0.4 to 5.1 hr). After repeated dosing, T1/2 was 2.5 ± 0.4 hr (range: 0.7 to 4.2 hr). AUC was 796 ± 159 ng∙hr/mL after the first dose and 818± 170 ng∙hr/mL after repeated dosing. Zolpidem was not hemodialyzable. No accumulation of unchanged drug appeared after 14 or 21 days. Zolpidem pharmacokinetics were not significantly different in renally-impaired patients. No dosage adjustment is necessary in patients with compromised renal function. However, as a general precaution, these patients should be closely monitored.
Controlled trials supporting safety and efficacy
Ambien CR was evaluated in two placebo-controlled studies for the treatment of patients with chronic primary insomnia (as defined in the APA Diagnostic and Statistical Manual of Mental Disorders, DSM IV).
Adult outpatients (18–64 years) with primary insomnia (N=212) were evaluated in a double-blind, randomized, parallel-group, 3-week trial comparing Ambien CR 12.5 mg and placebo. Ambien CR 12.5 mg decreased wake time after sleep onset (WASO) for the first 7 hours during the first 2 nights and for the first 5 hours after 2 weeks of treatment. Ambien CR 12.5 mg was superior to placebo on objective measures (polysomnography recordings) of sleep induction (by decreasing latency to persistent sleep [LPS]) during the first 2 nights of treatment and after 2 weeks of treatment. Ambien CR 12.5 mg was also superior to placebo on the patient reported global impression regarding the aid to sleep after the first 2 nights and after 3 weeks of treatment.
Elderly outpatients (≥65 years) with primary insomnia (N=205) were evaluated in a double-blind, randomized, parallel-group, 3-week trial comparing Ambien CR 6.25 mg and placebo. Ambien CR 6.25 mg decreased wake time after sleep onset (WASO) for the first 6 hours during the first 2 nights and the first 4 hours after 2 weeks of treatment. Ambien CR 6.25 mg was superior to placebo on objective measures (polysomnography recordings) of sleep induction (by decreasing latency to persistent sleep [LPS]) during the first 2 nights of treatment and after 2 weeks on treatment. Ambien CR 6.25 mg was superior to placebo on the patient reported global impression regarding the aid to sleep after the first 2 nights and after 3 weeks of treatment.
In both studies, in patients treated with Ambien CR, polysomnography showed increased wakefulness at the end of the night compared to placebo-treated patients.
Studies Pertinent To Safety Concerns For Sedative/Hypnotic Drugs
Next-day residual effects
In five clinical studies; three controlled studies in adults (18–64 years of age) administered Ambien CR 12.5 mg and two controlled studies in the elderly (≥ 65 years of age) administered Ambien CR 6.25 mg or 12.5 mg, the effect of Ambien CR on vigilance, memory, or motor function were assessed using neurocognitive tests. In these studies, no significant decrease in performance was observed eight hours after a nighttime dose. In addition, no evidence of next-day residual effects were detected with Ambien CR 12.5 mg and 6.25 mg using self-ratings of sedation.
Next day somnolence was reported by 15% of the adult patients who received 12.5 mg Ambien CR versus 2% of the placebo group. Next day somnolence was reported by 6% of the elderly patients who received 6.25 mg Ambien CR versus 5% of the placebo group. (See Adverse Reactions.)
Rebound effects
Rebound insomnia, defined as a dose-dependent worsening in sleep parameters (latency, sleep efficiency, and number of awakenings) compared with baseline following discontinuation of treatment, is observed with short- and intermediate-acting hypnotics. In the two placebo-controlled studies in patients with primary insomnia, a rebound effect was only observed on the first night after abrupt discontinuation of Ambien CR. On the second night, there was no worsening compared to baseline in the Ambien CR group.
INDICATIONS AND USAGE
Ambien CR (zolpidem tartrate extended-release tablets) is indicated for the treatment of insomnia, characterized by difficulties with sleep onset and/or sleep maintenance (as measured by wake time after sleep onset). (See Clinical Pharmacology: Controlled trials supporting safety and efficacy.)
The clinical trials performed in support of efficacy were both 3 weeks in duration, although the final formal assessments of sleep latency and maintenance were performed after 2 weeks of treatment.
CONTRAINDICATIONS
Ambien CR is contraindicated in patients with known hypersensitivity to zolpidem tartrate or to any of the inactive ingredients in the formulation.
WARNINGS
Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative/hypnotic drugs, including zolpidem. Because some of the important adverse effects of zolpidem appear to be dose related (see Precautions and Dosage and Administration), it is important to use the smallest possible effective dose, especially in the elderly.
A variety of abnormal thinking and behavior changes have been reported to occur in association with the use of sedative/hypnotics. Some of these changes may be characterized by decreased inhibition (e.g., aggressiveness and extroversion that seemed out of character), similar to effects produced by alcohol and other CNS depressants. Visual and auditory hallucinations have been reported as well as behavioral changes such as bizarre behavior, agitation, and depersonalization. Complex behaviors such as “sleep-driving” (i.e., driving while not fully awake after ingesting a sedative-hypnotic, with amnesia for the event) have been reported. These events can occur in sedative-hypnotic-nave as well as in sedative-hypnotic-experienced persons. Although behaviors such as sleep driving may occur with zolpidem alone at therapeutic doses, the use of alcohol and other CNS depressants with zolpidem appears to increase the risk of such behaviors, as does the use of zolpidem at doses exceeding the maximum recommended dose. Due to the risk to the patient and the community, discontinuation of zolpidem should be strongly considered for patients who report a “sleep-driving” episode. Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events. Amnesia, anxiety and other neuro-psychiatric symptoms may occur unpredictably. In primarily depressed patients, worsening of depression, including suicidal thinking, has been reported in association with the use of sedative-hypnotics.
It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
Following the rapid dose decrease or abrupt discontinuation of sedative/ hypnotics, there have been reports of signs and symptoms similar to those associated with withdrawal from other CNS-depressant drugs (see Drug Abuse and Dependence).
Zolpidem, like other sedative/hypnotic drugs, has CNS-depressant effects. Due to the rapid onset of action, Ambien CR should only be ingested immediately prior to going to bed. Patients should be cautioned against engaging in hazardous occupations requiring complete mental alertness or motor coordination such as operating machinery or driving a motor vehicle after ingesting the drug, including potential impairment of the performance of such activities that may occur the day following ingestion of Ambien CR. Zolpidem showed additive effects when combined with alcohol and should not be taken with alcohol. Patients should also be cautioned about possible combined effects with other CNS-depressant drugs. Dosage adjustments may be necessary when Ambien CR is administered with such agents because of the potentially additive effects.
Severe anaphylactic and anaphylactoid reactions
Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including zolpidem. Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis. Some patients have required medical therapy in the emergency department. If angioedema involves the tongue, glottis, or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with zolpidem should not be rechallenged with the drug.
PRECAUTIONS
General
Use in the elderly and/or debilitated patients
Impaired motor and/or cognitive performance after repeated exposure or unusual sensitivity to sedative/hypnotic drugs is a concern in the treatment of elderly and/or debilitated patients. Therefore, the recommended Ambien CR dosage is 6.25 in such patients (see Dosage and Administration) to decrease the possibility of side effects. These patients should be closely monitored.
Use in patients with concomitant illness
Clinical experience with zolpidem in patients with concomitant systemic illness is limited. Caution is advisable in using Ambien CR in patients with diseases or conditions that could affect metabolism or hemodynamic responses. Although studies did not reveal respiratory depressant effects at hypnotic doses of zolpidem tartrate in normals or in patients with mild to moderate chronic obstructive pulmonary disease (COPD), a reduction in the Total Arousal Index together with a reduction in lowest oxygen saturation and increase in the times of oxygen desaturation below 80% and 90% was observed in patients with mild-to-moderate sleep apnea when treated with an immediate-release formulation of zolpidem tartrate (10 mg) when compared to placebo. However, precautions should be observed if Ambien CR is prescribed to patients with compromised respiratory function, since sedative/hypnotics have the capacity to depress respiratory drive. Post-marketing reports of respiratory insufficiency in patients receiving immediate-release zolpidem tartrate, most of which involved patients with pre-existing respiratory impairment, have been received. Data in end-stage renal failure patients repeatedly treated with immediate-release zolpidem tartrate did not demonstrate drug accumulation or alterations in pharmacokinetic parameters. No dosage adjustment in renally impaired patients is required; however, these patients should be closely monitored (see Pharmacokinetics). A study in subjects with hepatic impairment did reveal prolonged elimination in this group; therefore, treatment should be initiated with Ambien CR 6.25 mg in patients with hepatic compromise, and they should be closely monitored.
Use in depression
Sedative/hypnotic drugs should be administered with caution to patients exhibiting signs or symptoms of depression. Suicidal tendencies may be present in such patients and protective measures may be required. Intentional overdosage is more common in this group of patients; therefore, the least amount of drug that is feasible should be prescribed for the patient at any one time.
Information for Patients
Patient information is printed at the end of this insert. To assure safe and effective use of Ambien CR, this information and instructions provided in the patient information section should be discussed with patients.
“Sleep-Driving” and other complex behaviors: There have been reports of people getting out of bed after taking a sedative-hypnotic and driving their cars while not fully awake, often with no memory of the event. If a patient experiences such an episode, it should be reported to his or her doctor immediately, since “sleep-driving” can be dangerous. This behavior is more likely to occur when Ambien CR is taken with alcohol or other central nervous system depressants (see Warnings). Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events.
Laboratory Tests
There are no specific laboratory tests recommended.
Drug interactions
CNS-active drugs
An immediate-release formulation of zolpidem tartrate was evaluated in healthy volunteers in single-dose interaction studies for several CNS drugs. A study involving haloperidol and zolpidem tartrate revealed no effect of haloperidol on the pharmacokinetics or pharmacodynamics of zolpidem. Imipramine in combination with zolpidem tartrate produced no pharmacokinetic interaction other than a 20% decrease in peak levels of imipramine, but there was an additive effect of decreased alertness. Similarly, chlorpromazine in combination with zolpidem tartrate produced no pharmacokinetic interaction, but there was an additive effect of decreased alertness and psychomotor performance. The lack of a drug interaction following single-dose administration does not predict a lack following chronic administration.
An additive effect on psychomotor performance between alcohol and zolpidem tartrate was demonstrated.
A single-dose interaction study with zolpidem tartrate 10 mg and fluoxetine 20 mg at steady-state levels in male volunteers did not demonstrate any clinically significant pharmacokinetic or pharmacodynamic interactions. When multiple doses of zolpidem tartrate and fluoxetine at steady-state concentrations were evaluated in healthy females, the only significant change was a 17% increase in the zolpidem half-life. There was no evidence of an additive effect in psychomotor performance.
Following five consecutive nightly doses of zolpidem tartrate 10 mg in the presence of sertraline 50 mg (17 consecutive daily doses, at 7:00 am, in healthy female volunteers), zolpidem Cmax was significantly higher (43%) and Tmax was significantly decreased (53%). Pharmacokinetics of sertraline and N-desmethylsertraline were unaffected by zolpidem.
Since the systematic evaluations of Ambien CR in combination with other CNS-active drugs have been limited, careful consideration should be given to the pharmacology of any CNS-active drug to be used with zolpidem. Any drug with CNS-depressant effects could potentially enhance the CNS-depressant effects of zolpidem.
Drugs that affect drug metabolism via cytochrome P450
A randomized, double-blind, crossover interaction study in ten healthy volunteers between itraconazole (200 mg once daily for 4 days) and a single dose of an immediate-release formulation of zolpidem tartrate (10 mg) given five hours after the last dose of itraconazole resulted in a 34% increase in AUC0»∞ of zolpidem. There were no significant pharmacodynamic effects of zolpidem on subjective drowsiness, postural sway, or psychomotor performance.
A randomized, placebo-controlled, crossover interaction study in eight healthy female volunteers between five consecutive daily doses of rifampin (600 mg) and a single dose of an immediate-release formulation of zolpidem tartrate (20 mg) given 17 hours after the last dose of rifampin showed significant reductions of the AUC (−73%), Cmax (−58%), and T1/2 (−36%) of zolpidem together with significant reductions in the pharmacodynamic effects of zolpidem.
Other drugs
A study involving cimetidine/zolpidem tartrate and ranitidine/zolpidem tartrate combinations revealed no effect of either drug on the pharmacokinetics or pharmacodynamics of zolpidem. Zolpidem had no effect on digoxin kinetics and did not affect prothrombin time when given with warfarin in normal subjects. Zolpidem's sedative/hypnotic effect was reversed by flumazenil; however, no significant alterations in zolpidem pharmacokinetics were found.
Drug/Laboratory test interactions
Zolpidem is not known to interfere with commonly employed clinical laboratory tests. In addition, clinical data indicate that zolpidem does not cross-react with benzodiazepines, opiates, barbiturates, cocaine, cannabinoids, or amphetamines in two standard urine drug screens.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenesis
Zolpidem tartrate was administered to CD-1 mice and Sprague-Dawley rats for two years at dietary dosages of 4, 18, and 80 mg/kg/day. No evidence of carcinogenic potential was observed in either mice or rats at doses up to 80 mg base/kg/day (40 and 80 times the maximum recommended human dose [MHRD] of Ambien CR 12.5 mg [10 mg zolpidem base], respectively, on a mg/m2 basis).
Mutagenesis
Zolpidem did not have mutagenic activity in several tests including an in vitro bacterial reverse mutation (Ames) assay, an in vitro mammalian gene forward mutation assay in mouse lymphoma cells, and an in vitro unscheduled DNA synthesis in rat hepatocytes. Zolpidem was not clastogenic in an in vitro chromosomal aberration assay in human lymphocytes or in an in vivo micronucleus test in mice.
Impairment of Fertility
Zolpidem tartrate was administered by oral gavage to Sprague-Dawley rats at doses of 4, 20, or 100 mg base/kg/day. Treatment of males began 71 days prior to mating and continued through mating while treatment of females began 14 days prior to mating and continued through mating, gestation, and weaning which occurred on post partum Day 25. Zolpidem administered at 100 mg base/kg was associated with irregular estrus cycles and prolonged pre-coital intervals, but did not produce a decline in fertility. The no-effect dose was 20 mg base/kg/day (20 times the MRHD of Ambien CR on a mg/m2 basis).
Pregnancy
Teratogenic Effects
Pregnancy Category C.
Zolpidem tartrate was administered to pregnant Sprague-Dawley rats by oral gavage during the period of organogenesis at doses of 4, 20, or 100 mg based/kg/day. Adverse maternal and embryo/fetal effects occurred at doses of 20 mg base/kg and higher, manifesting as dose-related lethargy and ataxia in pregnant rats while examination of fetal skull bones revealed a dose-related trend toward incomplete ossification. Teratogenicity was not observed at any dose level. The no-effect dose of zolpidem for maternal and embryo/fetal toxicity was 4 mg base/kg/day (4 times the MRHD of Ambien CR on a mg/m2 basis).
Administration of zolpidem tartrate to pregnant Himalayan Albino rabbits at doses of 1, 4, or 16 mg base/kg/day by oral gavage (up to 30 times the MRHD of Ambien CR, on a mg/m2 basis) during the period of organogenesis produced dose-related maternal sedation and decreased maternal body weight gain at all doses. At the high dose of 16 mg base/kg, there was an increase in postimplantation fetal loss and under-ossification of sternebrae in viable fetuses. Teratogenicity was not observed at any dose level. The no-effect dose of zolpidem for maternal toxicity was below 1 mg base/kg/day (< 2-times the MRHD of Ambien CR, on a mg/m2 basis). The no-effect dose for embryofetal toxicity was 4 mg base/kg/day (8 times the MRHD of Ambien CR on a mg/m2 basis).
Administration of zolpidem tartrate at doses of 4, 20, or 100 mg base/kg/day to pregnant Sprague-Dawley rats starting on Day 15 of gestation and continuing through Day 21 of the postnatal lactation period produced dose-dependent lethargy and ataxia in dams at doses of 20 mg base/kg and higher. Decreased maternal body weight gain as well as evidence on non-secreting mammary glands and a single incidence of maternal death was observed at 100 mg base/kg. Effects observed on rat pups included decreased body weight with maternal doses of 20 mg base/kg and higher and decreased pup survival at maternal doses of 100 mg base/kg. The no-effect dose for maternal and offspring toxicity was 4 mg base/kg (4 times the MRHD of Ambien CR on a mg/m2 basis).
There are no adequate and well-controlled studies in pregnant women. Ambien CR should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
Studies to assess the effects on children whose mothers took zolpidem during pregnancy have not been conducted. However, children born of mothers taking sedative/hypnotic drugs may be at some risk for withdrawal symptoms from the drug during the postnatal period. In addition, neonatal flaccidity has been reported in infants born of mothers who received sedative/hypnotic drugs during pregnancy.
Labor And Delivery
Ambien CR has no established use in labor and delivery. (See also Pregnancy.)
Nursing Mothers
Studies in lactating mothers indicate that the half-life of zolpidem is similar to that in young normal volunteers (2.6± 0.3 hr). Between 0.004% and 0.019% of the total administered dose is excreted into milk, but the effect of zolpidem on the infant is unknown.
In addition, in a rat study, zolpidem inhibited the secretion of milk. The no-effect dose was 4 mg base/kg or 6 times the recommended human dose in mg/m2.
The use of Ambien CR in nursing mothers is not recommended.
Pediatric Use
Safety and effectiveness of Ambien CR in patients below the age of 18 have not been established.
Geriatric Use
A total of 99 elderly (≥65 years of age) received daily doses of 6.25 mg Ambien CR in a 3-week placebo-controlled study. The adverse event profile of Ambien CR 6.25 mg in this population was similar to that of Ambien CR 12.5 mg in younger adults (≤ 64 years of age). Dizziness was reported in 8% of Ambien CR-treated patients compared with 3% of those treated with placebo.
ADVERSE REACTIONS
Associated with discontinuation of treatment
In clinical trials with Ambien CR, 3.5% of 201 patients receiving 6.25-mg or 12.5-mg of Ambien CR discontinued treatment because of an adverse event. Events most commonly associated with discontinuation were somnolence (1.0%) and dizziness (1.0%).
Data from a clinical study in which selective serotonin reuptake inhibitor (SSRI)-treated patients were given immediate-release zolpidem tartrate revealed that four of the seven discontinuations during double-blind treatment with zolpidem (n=95) were associated with impaired concentration, continuing or aggravated depression, and manic reaction; one patient treated with placebo (n =97) was discontinued after an attempted suicide.
Incidence in controlled clinical trials
Most commonly observed adverse events in controlled trials
During treatment with Ambien CR in adults and elderly at daily doses of 12.5 mg and 6.25 mg, respectively, each for three weeks, the most commonly observed adverse events associated with the use of Ambien CR were headache, somnolence, and dizziness.
Adverse events observed at an incidence of ≥1% in controlled trials of Ambien CR
The following tables enumerate treatment-emergent adverse event frequencies that were observed at an incidence equal to 1% or greater among patients with insomnia who received Ambien CR in placebo-controlled trials. Events reported by investigators were classified utilizing the MedDRA dictionary for the purpose of establishing event frequencies. The prescriber should be aware that these figures cannot be used to predict the incidence of side effects in the course of usual medical practice in which patient characteristics and other factors differ from those that prevailed in these clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigators involving related drug products and uses, since each group of drug trials is conducted under a different set of conditions. However, the cited figures provide the physician with a basis for estimating the relative contribution of drug and nondrug factors to the incidence of side effects in the population studied.
The following tables were derived from results of two placebo-controlled efficacy trials involving Ambien CR. These trials involved patients with primary insomnia who were treated for 3 weeks with Ambien CR at doses of 12.5 mg (Table 1) or 6.25 mg (Table 2), respectively. The tables include only adverse events occurring at an incidence of at least 1% for Ambien CR patients and with an incidence greater than that seen in the placebo patients.
| Body System/Adverse Event * | Ambien CR 12.5 mg (N = 102) |
Placebo (N = 110) |
|---|---|---|
| Infections and infestations | ||
| Influenza | 3 | 0 |
| Gastroenteritis | 1 | 0 |
| Labyrinthitis | 1 | 0 |
| Metabolism and nutrition disorders | ||
| Appetite disorder | 1 | 0 |
| Psychiatric disorders | ||
| Hallucinations † | 4 | 0 |
| Disorientation | 3 | 2 |
| Anxiety | 2 | 0 |
| Depression | 2 | 0 |
| Psychomotor retardation | 2 | 0 |
| Binge eating | 1 | 0 |
| Depersonalization | 1 | 0 |
| Disinhibition | 1 | 0 |
| Euphoric mood | 1 | 0 |
| Mood swings | 1 | 0 |
| Stress symptoms | 1 | 0 |
| Nervous system disorders | ||
| Headache | 19 | 16 |
| Somnolence | 15 | 2 |
| Dizziness | 12 | 5 |
| Memory disorders ‡ | 3 | 0 |
| Balance disorder | 2 | 0 |
| Disturbance in attention | 2 | 0 |
| Hypoesthesia | 2 | 1 |
| Ataxia | 1 | 0 |
| Paresthesia | 1 | 0 |
| Eye disorders | ||
| Visual disturbance | 3 | 0 |
| Eye redness | 2 | 0 |
| Vision blurred | 2 | 1 |
| Altered visual depth perception | 1 | 0 |
| Asthenopia | 1 | 0 |
| Ear and labyrinth disorders | ||
| Vertigo | 2 | 0 |
| Tinnitus | 1 | 0 |
| Respiratory, thoracic and mediastinal disorders | ||
| Throat irritation | 1 | 0 |
| Gastrointestinal disorders | ||
| Nausea | 7 | 4 |
| Constipation | 2 | 0 |
| Abdominal discomfort | 1 | 0 |
| Abdominal tenderness | 1 | 0 |
| Frequent bowel movements | 1 | 0 |
| Gastroesophageal reflux disease | 1 | 0 |
| Vomiting | 1 | 0 |
| Skin and subcutaneous tissue disorders |
||
| Rash | 1 | 0 |
| Skin wrinkling | 1 | 0 |
| Urticaria | 1 | 0 |
| Musculoskeletal and connective tissue disorders | ||
| Back pain | 4 | 3 |
| Myalgia | 4 | 0 |
| Neck pain | 1 | 0 |
| Reproductive system and breast disorders | ||
| Menorrhagia | 1 | 0 |
| General disorders and administration site conditions | ||
| Fatigue | 3 | 2 |
| Asthenia | 1 | 0 |
| Chest discomfort | 1 | 0 |
| Investigations | ||
| Blood pressure increased | 1 | 0 |
| Body temperature increased | 1 | 0 |
| Injury, poisoning and procedural complications | ||
| Contusion | 1 | 0 |
| Social circumstances | ||
| Exposure to poisonous plant | 1 | 0 |
| Body System/Adverse Event * | Ambien CR 6.25 mg (N=99) |
Placebo (N=106) |
|---|---|---|
| Infections and infestations | ||
| Nasopharyngitis | 6 | 4 |
| Lower respiratory tract infection | 1 | 0 |
| Otitis externa | 1 | 0 |
| Upper respiratory tract infection | 1 | 0 |
| Psychiatric disorders | ||
| Anxiety | 3 | 2 |
| Psychomotor retardation | 2 | 0 |
| Apathy | 1 | 0 |
| Depressed mood | 1 | 0 |
| Nervous system disorders | ||
| Headache | 14 | 11 |
| Dizziness | 8 | 3 |
| Somnolence | 6 | 5 |
| Burning sensation | 1 | 0 |
| Dizziness postural | 1 | 0 |
| Memory disorders † | 1 | 0 |
| Muscle contractions involuntary | 1 | 0 |
| Paresthesia | 1 | 0 |
| Tremor | 1 | 0 |
| Cardiac disorders | ||
| Palpitations | 2 | 0 |
| Respiratory, thoracic and mediastinal disorders | ||
| Dry throat | 1 | 0 |
| Gastrointestinal disorders | ||
| Flatulence | 1 | 0 |
| Vomiting | 1 | 0 |
| Skin and subcutaneous tissue disorders | ||
| Rash | 1 | 0 |
| Urticaria | 1 | 0 |
| Musculoskeletal and connective tissue disorders | ||
| Arthralgia | 2 | 0 |
| Muscle cramp | 2 | 1 |
| Neck pain | 2 | 0 |
| Renal and urinary disorders | ||
| Dysuria | 1 | 0 |
| Reproductive system and breast disorders | ||
| Vulvovaginal dryness | 1 | 0 |
| General disorders and administration site conditions | ||
| Influenza like illness | 1 | 0 |
| Pyrexia | 1 | 0 |
| Injury, poisoning and procedural complications | ||
| Neck injury | 1 | 0 |
Dose relationship for adverse events
There is evidence from dose comparison trials suggesting a dose relationship for many of the adverse events associated with zolpidem use, particularly for certain CNS and gastrointestinal adverse events.
Other Adverse Events Observed During the Premarketing Evaluation of Ambien CR
Other treatment-emergent adverse events associated with participation in Ambien CR studies (those reported at frequencies of <1%) were not different in nature or frequency to those seen in studies with immediate-release zolpidem tartrate, which are listed below.
Adverse Events Observed During the Premarketing Evaluation of Immediate-Release Zolpidem Tartrate
Immediate-release zolpidem tartrate, was administered to 3,660 subjects in clinical trials throughout the U.S., Canada, and Europe. Treatment-emergent adverse events associated with clinical trial participation were recorded by clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals experiencing treatment-emergent adverse events, similar types of untoward events were grouped into a smaller number of standardized event categories and classified utilizing a modified World Health Organization (WHO) dictionary of preferred terms. The frequencies presented, therefore, represent the proportions of the 3,660 individuals exposed to zolpidem, at all doses, who experienced an event of the type cited on at least one occasion while receiving immediate-release zolpidem. All reported treatment-emergent adverse events are included, except those coding terms that are so general as to be uninformative and those events where a drug cause was remote. It is important to emphasize that, although the events reported did occur during treatment with immediate-release zolpidem, they were not necessarily caused by it.
Adverse events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring in greater than 1/100 subjects; infrequent adverse events are those occurring in 1/100 to 1/1,000 patients; rare events are those occurring in less than 1/1,000 patients.
Autonomic nervous system: Frequent: dry mouth. Infrequent: increased sweating, pallor, postural hypotension, syncope. Rare: abnormal accommodation, altered saliva, flushing, glaucoma, hypotension, impotence, increased saliva, tenesmus.
Body as a whole: Frequent: allergy, asthenia, back pain, influenza-like symptoms. Infrequent: chest pain, edema, falling, fatigue, fever, malaise, trauma. Rare: allergic reaction, allergy aggravated, anaphylactic shock, face edema, hot flashes, increased ESR, pain, restless legs, rigors, tolerance increased, weight decrease.
Cardiovascular system: Frequent: palpitation. Infrequent: cerebrovascular disorder, hypertension, tachycardia. Rare: angina pectoris, arrhythmia, arteritis, circulatory failure, extrasystoles, hypertension aggravated, myocardial infarction, phlebitis, pulmonary embolism, pulmonary edema, varicose veins, ventricular tachycardia.
Central and peripheral nervous system: Frequent: ataxia, confusion, depression, dizziness, drowsiness, drugged feeling, euphoria, headache, insomnia, lethargy, lightheadedness, vertigo. Infrequent: abnormal dreams, agitation, amnesia, anxiety, decreased cognition, detached, difficulty concentrating, dysarthria, emotional lability, hallucination, hypoesthesia, illusion, leg cramps, migraine, nervousness, paresthesia, sleep disorder, sleeping (after daytime dosing), speech disorder, stupor, tremor. Rare: abnormal gait, abnormal thinking, aggressive reaction, apathy, appetite increased, decreased libido, delusion, dementia, depersonalization, dysphasia, feeling strange, hypokinesia, hypotonia, hysteria, intoxicated feeling, manic reaction, neuralgia, neuritis, neuropathy, neurosis, panic attacks, paresis, personality disorder, somnambulism, suicide attempts, tetany, yawning.
Gastrointestinal system: Frequent: abdominal pain, diarrhea, dyspepsia, hiccup, nausea. Infrequent: anorexia, constipation, dysphagia, flatulence, gastroenteritis, vomiting.Rare: enteritis, eructation, esophagospasm, gastritis, hemorrhoids, intestinal obstruction, rectal hemorrhage, tooth caries.
Hematologic and lymphatic system: Rare: anemia, hyperhemoglobinemia, leukopenia, lymphadenopathy, macrocytic anemia, purpura, thrombosis.
Immunologic system: Infrequent: infection. Rare: abscess, herpes simplex, herpes zoster, otitis externa, otitis media.
Liver and biliary system: Infrequent: abnormal hepatic function, increased SGPT. Rare: bilirubinemia, increased SGOT.
Metabolic and nutritional: Infrequent: hyperglycemia, thirst. Rare: gout, hypercholesteremia, hyperlipidemia, increased alkaline phosphatase, increased BUN, periorbital edema.
Musculoskeletal system: Frequent: arthralgia, myalgia. Infrequent: arthritis. Rare: arthrosis, muscle weakness, sciatica, tendinitis.
Reproductive system: Infrequent: menstrual disorder, vaginitis.Rare: breast fibroadenosis, breast neoplasm, breast pain.
Respiratory system: Frequent: pharyngitis, sinusitis, upper respiratory infection. Infrequent: bronchitis, coughing, dyspnea, rhinitis. Rare: bronchospasm, epistaxis, hypoxia, laryngitis, pneumonia.
Skin and appendages: Frequent: rash. Infrequent: pruritus. Rare: acne, bullous eruption, dermatitis, furunculosis, injection-site inflammation, photosensitivity reaction, urticaria.
Special senses: Frequent: diplopia, vision abnormal. Infrequent: eye irritation, eye pain, scleritis, taste perversion, tinnitus. Rare: conjunctivitis, corneal ulceration, lacrimation abnormal, parosmia, photopsia.
Urogenital system: Frequent: urinary tract infection. Infrequent: cystitis, urinary incontinence.Rare: acute renal failure, dysuria, micturition frequency, nocturia, polyuria, pyelonephritis, renal pain, urinary retention.
DRUG ABUSE AND DEPENDENCE
Controlled substance
Zolpidem tartrate is classified as a Schedule IV controlled substance under the controlled Substances Act. Examples of other drugs placed in Schedule IV include benzodiazepines (diazepam, alprazolam, etc) and the non-benzodiazepine hypnotics (zaleplon and eszopiclone).
Abuse and dependence
Abuse and addiction are separate and distinct from physical dependence and tolerance. Abuse is characterized by misuse of the drug for non-medical purposes, often in combination with other psychoactive substances. Physical dependence is a state of adaptation that is manifested by a specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug and/or administration of an antagonist. Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time. Tolerance may occur to both desired and undesired effects of drugs and may develop at different rates for different effects.
Addiction is a primary, chronic, neurobiological disease with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, using a multidisciplinary approach, but relapse is common.
Studies of abuse potential in former drug abusers found that the effects of single doses of an immediate-release formulation of zolpidem tartrate (Ambien) 40 mg were similar, but not identical, to diazepam 20 mg, while zolpidem tartrate 10 mg was difficult to distinguish from placebo.
Sedative/hypnotics have produced withdrawal signs and symptoms following abrupt discontinuation. These reported symptoms range from mild dysphoria and insomnia to a withdrawal syndrome that may include abdominal and muscle cramps, vomiting, sweating, tremors, and convulsions. The U.S. clinical trial experience from zolpidem does not reveal any clear evidence for withdrawal syndrome. Nevertheless, the following adverse events included in DSM-III-R criteria for uncomplicated sedative/hypnotic withdrawal were reported during U.S. clinical trials following placebo substitution occurring within 48 hours following last zolpidem treatment: fatigue, nausea, flushing, lightheadedness, uncontrolled crying, emesis, stomach cramps, panic attack, nervousness, and abdominal discomfort. These reported adverse events occurred at an incidence of 1% or less. However, available data cannot provide a reliable estimate of the incidence, if any, of dependence during treatment at recommended doses. Rare post-marketing reports of abuse, dependence and withdrawal have been received.
Because persons with a history of addiction to, or abuse of, drugs or alcohol are at increased risk for misuse, abuse and addiction of zolpidem, they should be monitored carefully when receiving zolpidem or any other hypnotic.
OVERDOSAGE
Signs and symptoms
In postmarketing reports of overdose with immediate-release zolpidem tartrate alone, impairment of consciousness has ranged from somnolence to light coma. There was one case each of cardiovascular and respiratory compromise. Individuals have fully recovered from zolpidem tartrate overdoses up to 400 mg (40 times the maximum recommended dose of the immediate-release product). Overdose cases involving multiple CNS-depressant agents, including zolpidem tartrate, have resulted in more severe symptomatology, including fatal outcomes.
Recommended treatment
General symptomatic and supportive measures should be used along with immediate gastric lavage where appropriate. Intravenous fluids should be administered as needed. Flumazenil may be useful. As in all cases of drug overdose, respiration, pulse, blood pressure, and other appropriate signs should be monitored and general supportive measures employed. Hypotension and CNS depression should be monitored and treated by appropriate medical intervention. Sedating drugs should be withheld following zolpidem tartrate overdosage, even if excitation occurs. The value of dialysis in the treatment of overdosage has not been determined, although hemodialysis studies in patients with renal failure receiving therapeutic doses have demonstrated that zolpidem is not dialyzable.
Poison control center
As with the management of all overdosage, the possibility of multiple drug ingestion should be considered. The physician may wish to consider contacting a poison control center for up-to-date information on the management of hypnotic drug product overdosage.
DOSAGE AND ADMINISTRATION
The dose of Ambien CR should be individualized.
Ambien CR is available as extended-release tablets containing 6.25 mg or 12.5 mg of zolpidem tartrate for oral administration. Ambien CR extended-release tablets should be swallowed whole, and not be divided, crushed, or chewed. The effect of Ambien CR may be slowed by ingestion with or immediately after a meal.
The recommended dose of Ambien CR for adults is 12.5 mg immediately before bedtime.
Elderly or debilitated patients may be especially sensitive to the effects of zolpidem. Patients with hepatic insufficiency do not clear the drug as rapidly as normals. The recommended dose of Ambien CR in these patients is 6.25 mg immediately before bedtime (see Precautions).
HOW SUPPLIED
Ambien CR 6.25 mg tablets are composed of two layers1 and are coated, pink, round, bi-convex, debossed with A~ on one side and supplied as:
NDC Number Size
0024-5501-31 bottle of 100
0024-5501-50 bottle of
500
0024-5501-10 carton
of 30 unit dose
0024-5501-34 carton
of 100 unit dose
Ambien CR 12.5-mg tablets are composed of two layers1 and are coated, blue, round, bi-convex, debossed with A~ on one side and supplied as:
NDC Number Size
0024-5521-31 bottle of 100
0024-5521-50 bottle of
500
0024-5521-10 carton
of 30 unit dose
0024-5521-34 carton
of 100 unit dose
- 1
- Layers are covered by the coating and are indistinguishable. The tablets are to be swallowed whole and should not be crushed, chewed, or divided.
Store between 15°–25° C (59°–77°F). Limited excursions permissible up to 30° C (86°F).
INFORMATION FOR PATIENTS TAKING AMBIEN CR
Your doctor has prescribed Ambien CR to help you sleep. The following information is intended to guide you in the safe use of this medicine. It is not meant to take the place of your doctor's instructions. If you have any questions about Ambien CR tablets be sure to ask your doctor or pharmacist.
Ambien CR is used to treat different types of sleep problems, such as:
- trouble falling asleep
- waking up often during the night
Some people may have more than one of these problems.
Ambien CR belongs to a group of medicines known as the "sedative/hypnotics", or simply, sleep medicines. There are many different sleep medicines available to help people sleep better. Sleep problems are usually temporary, requiring treatment for only a short time, usually 1 or 2 days up to 1 or 2 weeks. Some people have chronic sleep problems that may require more prolonged use of sleep medicine. However, you should not use these medicines for long periods without talking with your doctor about the risks and benefits of prolonged use.
SIDE EFFECTS
Most common side effects:
- headache
- somnolence (sleepiness)
- dizziness
You may find that these medicines make you sleepy during the day. How drowsy you feel depends upon how your body reacts to the medicine, which sleep medicine you are taking, and how large a dose your doctor has prescribed. Daytime drowsiness is best avoided by taking the lowest dose possible that will still help you sleep at night. Your doctor will work with you to find the dose of Ambien CR that is best for you.
To manage these side effects while you are taking this medicine:
- When you first start taking Ambien CR or any other sleep medicine until you know whether the medicine will still have some carryover effect in you the next day, use extreme care while doing anything that requires complete alertness, such as driving a car, operating machinery, or piloting an aircraft.
- NEVER drink alcohol while you are being treated with Ambien CR or any sleep medicine. Alcohol can increase the side effects of Ambien CR or any other sleep medicine.
- Do not take any other medicines without asking your doctor first. This includes medicines you can buy without a prescription. Some medicines can cause drowsiness and are best avoided while taking Ambien CR.
- Always take the exact dose of Ambien CR prescribed by your doctor. Never change your dose without talking to your doctor first.
SPECIAL CONCERNS
There are some special problems that may occur while taking sleep medicines.
Memory problems: Sleep medicines may cause a special type of memory loss or "amnesia." When this occurs, a person may not remember what has happened for several hours after taking the medicine. This is usually not a problem since most people fall asleep after taking the medicine.
Memory loss can be a problem, however, when sleep medicines are taken while traveling, such as during an airplane flight and the person wakes up before the effect of the medicine is gone. This has been called "traveler's amnesia."
Be sure to talk to your doctor if you think you are having memory problems. Although memory problems are not very common while taking Ambien CR, in most instances, they can be avoided if you take Ambien CR only when you are able to get a full night's sleep (7 to 8 hours) before you need to be active again.
Tolerance: When sleep medicines are used every night for more than a few weeks, they may lose their effectiveness to help you sleep. This is known as "tolerance". Sleep medicines should, in most cases, be used only for short periods of time, such as 1 or 2 days and generally no longer than 1 or 2 weeks. If your sleep problems continue, consult your doctor, who will determine whether other measures are needed to overcome your sleep problems.
Dependence: Sleep medicines can cause dependence, especially when these medicines are used regularly for longer than a few weeks or at high doses. Some people develop a need to continue taking their medicines. This is known as dependence or "addiction."
When people develop dependence, they may have difficulty stopping the sleep medicine. If the medicine is suddenly stopped, the body is not able to function normally and unpleasant symptoms may occur (see Withdrawal). They may find that they have to keep taking the medicines either at the prescribed dose or at increasing doses just to avoid withdrawal symptoms.
All people taking sleep medicines have some risk of becoming dependent on the medicine. However, people who have been dependent on alcohol or other drugs in the past may have a higher chance of becoming addicted to sleep medicines. This possibility must be considered before using these medicines for more than a few weeks.
If you have been addicted to alcohol or drugs in the past, it is important to tell your doctor before starting Ambien or any sleep medicine.
Withdrawal: Withdrawal symptoms may occur when sleep medicines are stopped suddenly after being used daily for a long time. In some cases, these symptoms can occur even if the medicine has been used for only a week or two.
In mild cases, withdrawal symptoms may include unpleasant feelings. In more severe cases, abdominal and muscle cramps, vomiting, sweating, shakiness, and rarely, seizures may occur. These more severe withdrawal symptoms are very uncommon.
Another problem that may occur when sleep medicines are stopped is known as "rebound insomnia." This means that a person may have more trouble sleeping the first few nights after the medicine is stopped than before starting the medicine. If you should experience rebound insomnia, do not get discouraged. This problem usually goes away on its own after 1 or 2 nights.
If you have been taking Ambien CR or any other sleep medicine for more than 1 or 2 weeks, do not stop taking it on your own. Always follow your doctor's directions.
Changes in behavior and thinking: Some people using sleep medicines have experienced unusual changes in their thinking and/or behavior. These effects are not common. However, they have included:
- more outgoing or aggressive behavior than normal
- confusion
- strange behavior
- agitation
- hallucinations
- worsening of depression
- suicidal thoughts
How often these effects occur depends on several factors, such as a person's general health, the use of other medicines, and which sleep medicine is being used.
It is also important to realize that it is rarely clear whether these behavior changes are caused by the medicine, an illness, or occur on their own. In fact, sleep problems that do not improve may be due to illnesses that were present before the medicine was used. If you or your family notice any changes in your behavior, or if you have any unusual or disturbing thoughts, call your doctor immediately.
“Sleep-Driving” and other complex behaviors: There have been reports of people getting out of bed after taking a sedative-hypnotic and driving their cars while not fully awake, often with no memory of the event. If you experiences such an event, it should be reported to your doctor immediately, since “sleep-driving” can be dangerous. This behavior is more likely to occur when Ambien CR is taken with alcohol or other drugs such as those for the treatment of depression or anxiety. Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in people who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, people usually do not remember these events.
Pregnancy: Sleep medicines may cause sedation of the unborn baby when used during the last weeks of pregnancy.
Be sure to tell your doctor if you are pregnant, if you are planning to become pregnant, or if you become pregnant while taking Ambien CR.
SAFE USE OF SLEEPING MEDICINES
To ensure the safe and effective use of Ambien CR or any other sleep medicine, you should observe the following cautions:
- Ambien CR is a prescription medicine and should be used ONLY as directed by your doctor. Follow your doctor's instructions about how to take, when to take, and how long to take Ambien CR. Ambien CR tablets should not be divided, crushed, or chewed, and must be swallowed whole.
- Never use Ambien CR or any other sleep medicine for longer than directed by your doctor.
- If you develop an allergic reaction such as rash, hives, shortness of breath or swelling of your tongue or throat when using Ambien CR or any other sleep medicine, discontinue Ambien CR or other sleep medicine immediately and contact your doctor.
- If you notice any unusual and/or disturbing thoughts or behavior during treatment with Ambien CR or any other sleep medicine, contact your doctor.
- Tell your doctor about any medicines you may be taking, including medicines you may buy without a prescription. You should also tell your doctor if you drink alcohol. DO NOT use alcohol while taking Ambien CR or any other sleep medicine.
- Do not take Ambien CR unless you are able to get a full night's sleep before you must be active again. For example, Ambien CR should not be taken on an overnight airplane flight of less than 7 to 8 hours since "traveler's amnesia" may occur.
- Do not increase the prescribed dose of Ambien CR or any other sleep medicine unless instructed by your doctor.
- When you first start taking Ambien CR or any other sleep medicine, until you know whether the medicine will still have some carryover effect in you the next day, use extreme care while doing anything that requires complete alertness, such as driving a car, operating machinery, or piloting an aircraft.
- Be aware that you may have more sleeping problems the first night after stopping Ambien CR or any other sleep medicine.
- Be sure to tell your doctor if you are pregnant, if you are planning to become pregnant, or if you become pregnant while taking Ambien CR or any other sleep medicine.
- As with all prescription medicines, never share Ambien CR or any other sleep medicine with anyone else. Always store Ambien CR or any other sleep medicine in the original container that you received it in and store it out of reach of children.
- Ambien CR works very quickly. You should only take Ambien CR right before going to bed and are ready to go to sleep.
sanofi-aventis U.S. LLC
Bridgewater, NJ 08807
Ambien CR™ CIV
(zolpidem tartrate extended-release tablets)
Rev March 2007
Copyright, sanofi-aventis U.S. LLC 2007
| Ambien CR (zolpidem tartrate) | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Ambien CR (zolpidem tartrate) | |||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
Revised: 03/2007sanofi-aventis U.S. LLC