LEADER SORE THROAT
-
phenol spray
Cardinal Health
----------
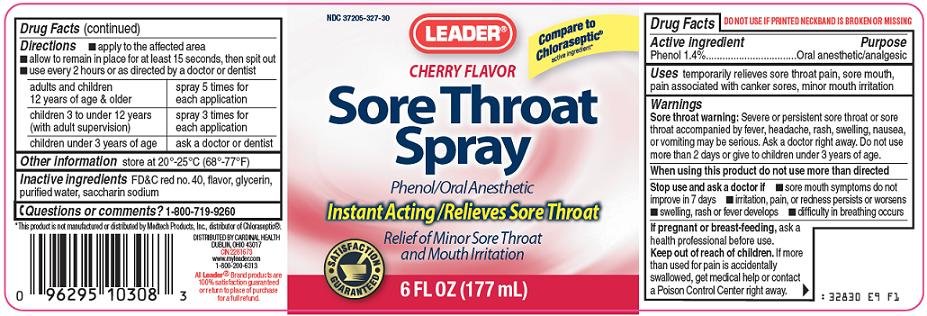
Cardinal Health Sore Throat Spray Drug FactsActive ingredient
Phenol 1.4%
Purpose
Oral anesthetic/analgesic
Uses
temporarily relieves sore throat pain, sore mouth, pain associated with canker sores, minor mouth irritation
Warnings
Sore throat warning: Severe or persistent sore throat or sore throat accompanied by fever, headache, rash, swelling, nausea, or vomiting may be serious. Ask a doctor right away. Do not use more than 2 days or give to children under 3 years of age.
When using this product
do not use more than directed
Stop use and ask a doctor if
- sore mouth symptoms do not improve in 7 days
- irritation, pain, or redness persists or worsens
- swelling, rash or fever develops
- difficulty in breathing occurs
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
If more than used for pain is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply to the affected area
- allow to remain in place for at least 15 seconds, then spit out
- use every 2 hours or as directed by a doctor or dentist
| adults and children 12 years of age & older | spray 5 times for each application |
| children 3 to under 12 years (with adult supervision) | spray 3 times for each application |
| children under 3 years of age | ask a doctor or dentist |
Other information
store at 20°-25°C (68°-77°F)
Inactive ingredients
FD&C red no. 40, flavor, glycerin, purified water, saccharin sodium
Questions or comments?
1-800-719-9260
Principal Display Panel
Compare to Chloraseptic® active ingredient
Cherry Flavor
Sore Throat Spray
Phenol/Oral Anesthetic
Instant Acting/Relieves Sore Throat
Relief of Minor Sore Throat and Mouth Irritation
Sore Throat Spray Label
| LEADER SORE THROAT
phenol spray |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part356 | 11/07/2000 | |
| Labeler - Cardinal Health (097537435) |