MEDI FIRST SINUS PAIN AND PRESSURE
-
acetaminophen and
phenylephrine hydrochloride tablet
MEDI FIRST PLUS SINUS PAIN AND PRESSURE
-
acetaminophen and
phenylephrine hydrochloride tablet
OTIS CLAPP MYGREX
-
acetaminophen and
phenylephrine hydrochloride tablet
Unifirst First Aid Corporation
----------
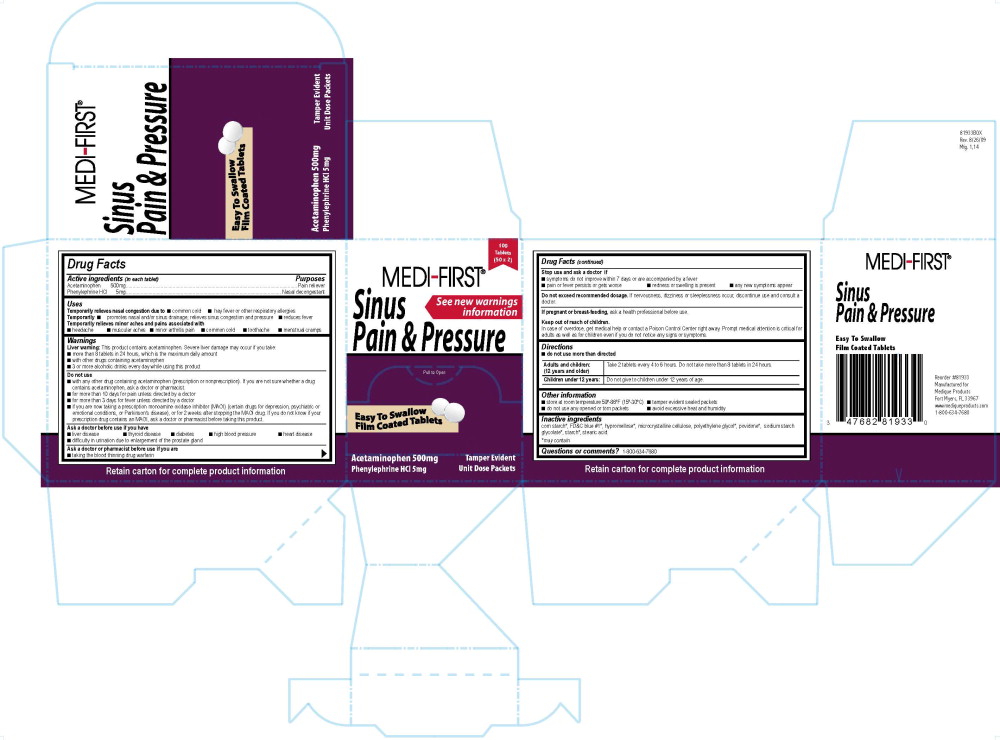
819R Sinus Pain and Pressure UniFirst MF/MFP OCDrug Facts
Active ingredients (in each tablet)
Acetaminophen 500mg
Phenylephrine HCl 5mg
Purposes
Pain reliever
Nasal decongestant
Uses
Temporarily relieves nasal congestion due to
- ■ common cold
- ■ hay fever or other respiratory allergies
Temporarily
- ■promotes nasal and/or sinus drainage; relieves sinus congestion and pressure
- ■ reduces fever
Temporarily relieves minor aches and pains associated with
- ■ headache
- ■ muscular aches
- ■ minor arthritis pain
- ■ common cold
- ■ toothache
- ■ menstrual cramps
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- ■ more than 8 tablets in 24 hours, which is the maximum daily amount
- ■ with other drugs containing acetaminophen
- ■ 3 or more alcoholic drinks every day while using this product
Do not use
- ■ with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- ■ for more than 10 days for pain unless directed by a doctor
- ■ for more than 3 days for fever unless directed by a doctor
- ■ if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- ■ liver disease
- ■ thyroid disease
- ■ diabetes
- ■ high blood pressure
- ■ heart disease
- ■ difficulty in urination due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
- ■ taking the blood thinning drug warfarin
Stop use and ask a doctor if
- ■ symptoms do not improve within 7 days or are accompanied by a fever
- ■ pain or fever persists or gets worse
- ■ redness or swelling is present
- ■ any new symptoms appear
Do not exceed recommended dosage. If nervousness, dizziness or sleeplessness occurs, discontinue use and consult a doctor.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- ■ do not use more than directed
Adults and children: (12 years and older) Take 2 tablets every 4 to 6 hours. Do not take more than 8 tablets in 24 hours.
Children under 12 years:Do not give to children under 12 years of age.
Other information
- ■ store at room temperature 59º-86ºF (15º-30ºC)
- ■ tamper evident sealed packets
- ■ do not use any opened or torn packets
- ■ avoid excessive heat and humidity
Inactive ingredients
corn starch*, FD&C blue #1*, hypromellose*, microcrystalline cellulose, polyethylene glycol*, povidone*, sodium starch glycolate*, starch*, stearic acid.
*may contain
Questions or comments? 1-800-634-7680
819R MF Sinus Pain and Pressure Label
100 Tablets
(50 x 2)
Medi-First®
Sinus Pain and Pressure
See new warnings information
Pull to Open
Easy To Swallow
Film Coated Tablets
Acetaminophen 500 mg
Phenylephrine HCl 5 mg
Tamper Evident
Unit Dose Packets
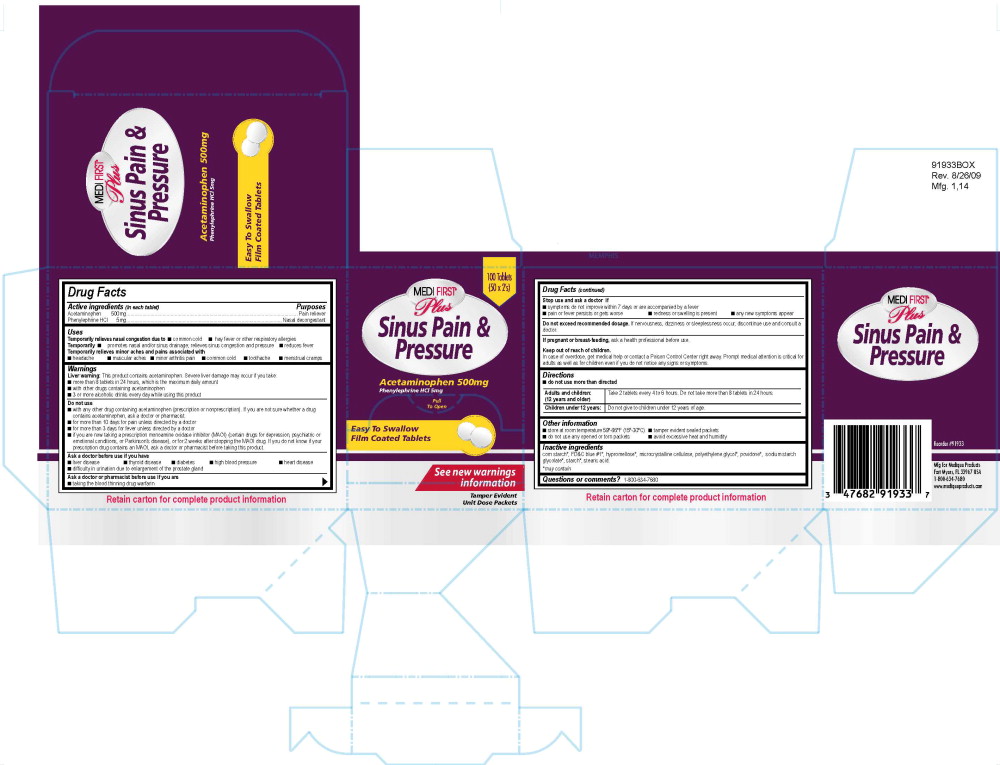
819R MFP Sinus Pain and Pressure Label
100 Tablets
(50 x 2's)
Medi First® Plus
Sinus Pain and Pressure
Acetaminophen 500 mg
Phenylephrine HCl 5 mg
Pull to Open
Easy To Swallow
Film Coated Tablets
See new warnings information
Tamper Evident Unit Dose Packets
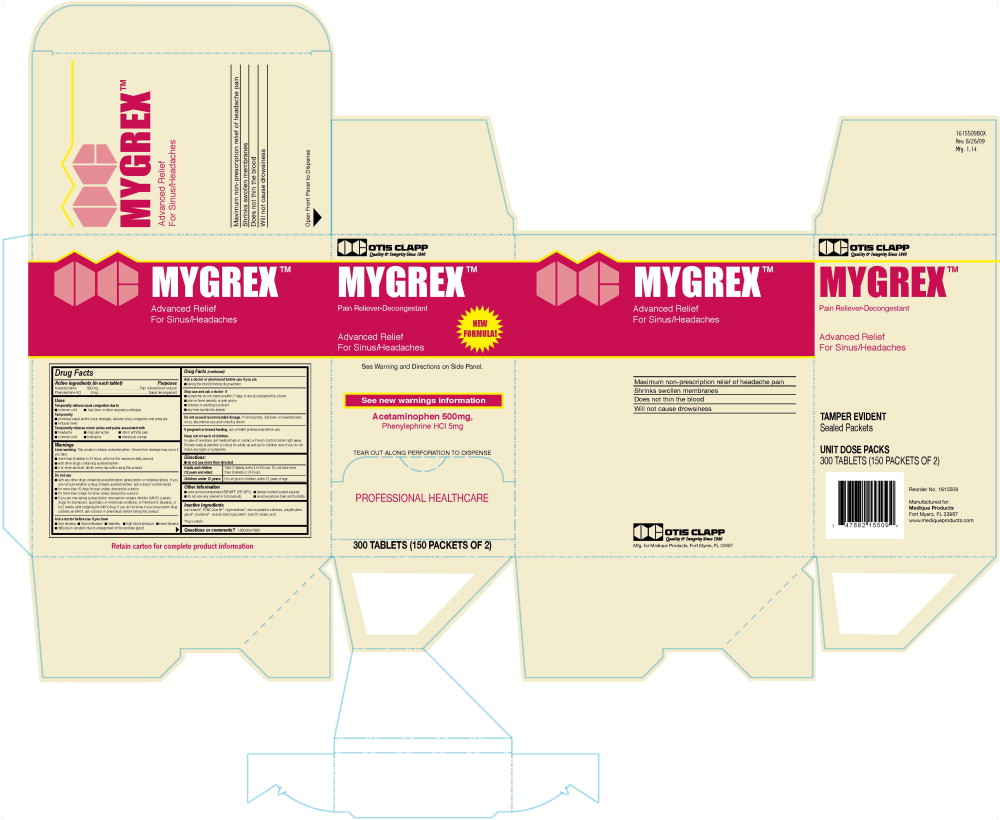
819R OC Mygrex Label
Otis Clapp
Quality and Integrity Since 1840
MYGREX ™
Pain Reliever-Decongestant
New Formula
Advanced Relief
For Sinus/Headaches
See Warnings and Directions on Side Panel
See new warnings information
Acetaminophen 500 mg,
Phenylephrine HCl 5 mg
Tear Out Along Perforation To Dispense
PROFESSIONAL HEALTHCARE
300 Tablets (150 PACKETS OF 2)
| MEDI FIRST SINUS PAIN AND PRESSURE
acetaminophen, phenylephrine hydrochloride tablet |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 12/30/2008 | |
| MEDI FIRST PLUS SINUS PAIN AND PRESSURE
acetaminophen, phenylephrine hydrochloride tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 12/30/2008 | |
| OTIS CLAPP MYGREX
acetaminophen, phenylephrine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 12/30/2008 | |
| Labeler - Unifirst First Aid Corporation (832947092) |