BISACODYL
-
bisacodyl tablet, delayed release
Sunrise Pharmaceutical Inc
----------
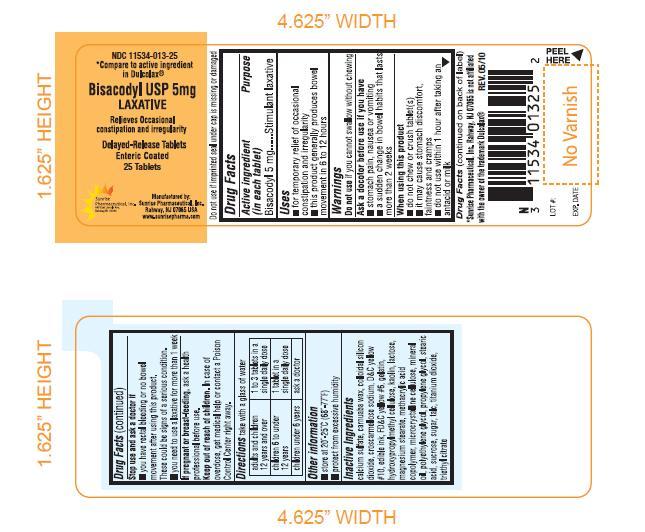
Bisacodyl USP 5 mg LaxativeACTIVE INGREDIENT
Bisacodyl USP 5mg.
PURPOSE
Stimulant laxative.
USE
For temporary relief of occasional constipation and irregularity
This product generally produces bowel movement in 6 to 12 hours.
WARNINGS
Do not use if you cannot swallow without chewing.
ASK DOCTOR BEFORE USE IF YOU HAVE
Stomach pain, nausea or vomiting
A sudden change in bowel habits that lasts for more than 2 weeks.
WHEN USING THIS PRODUCT
Do not chew or crush tablet(s).
It may cause stomach discomfort, faintness and cramps.
Do not use within 1 hour after taking an antacid or milk.
STOP USE AND ASK A DOCTOR IF
You have rectal bleeding or no bowel movement after using this product. These could be signs of serious condition.
You need to use laxative for more than 1 week
IF PREGNANT OR BREAST FEEDING
Ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS
Take with a glass of water
| Adults and children 12 years and over | 1 to 3 tablets in a single daily dose |
| Children 6 to under 12 years | 1 tablet in a single daily dose |
| Children under 6 years | Ask a doctor |
OTHER INFORMATION
Store at 20°-25°C(68°-77°F)
INACTIVE INGREDIENT
Calcium sulfate, carnuba wax, colloidal silicon dioxide, croscarmellose sodium, D & C yellow # 10, edible ink, FD & C yellow #6, gelatin, hydroxypropylymethyl cellulose, kaolin, lactose, magnesium stearate, methacrylic acid copolymer, microcrystalline celloluse, mineral oil, polyethylene glycol, propylene glycol, stearic acid, sucrose, sugar, talc, titanium dioxide, triethyl citrate.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
| BISACODYL
bisacodyl tablet, delayed release |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part334 | 07/08/2005 | |
| Labeler - Sunrise Pharmaceutical Inc (168522378) |