CHILDRENS QPAP
-
acetaminophen solution
Qualitest Pharmaceuticals
----------
Childrens Q-PAP Acetaminophen Liquid GrapeActive ingredient (in each 5 mL = 1 tsp)
Acetaminophen, USP 160 mg
Purpose
Pain reliever/ fever reducer
Uses
- temporarily reduces fever
- temporarily relieves minor aches and pains due to:
- the common cold
- flu
- headache
- sore throat
- toothache
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
Ask a doctor before use if your child has liver disease
Ask a doctor or pharmacist before use if your child is taking the blood thinning drug warfarin
When using this product do not exceed recommended dose.
Stop use and ask a doctor if
- pain gets worse or lasts more than 5 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222). Quick medical attention is critical even if you do not notice any signs or symptoms.
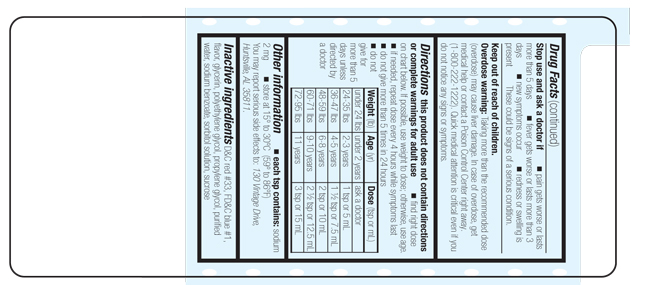
Directions
this product does not contain directions or complete warnings for adult use
- find right dose on chart below. If possible, use weight to dose; otherwise, use age.
- if needed, repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- do not give for more than 5 days unless directed by a doctor
| Weight (lb) | Age (yr) | Dose (tsp or mL) |
| under 24 lbs | under 2 years | ask a doctor |
| 24-35 lbs | 2-3 years |
1 tsp or 5 mL |
| 36-47 lbs | 4-5 years | 1 ½ tsp or 7.5 mL |
|
48-59 lbs | 6-8 years |
2 tsp or 10 mL |
| 60-71 lbs |
9-10 years | 2 ½ tsp or 12.5 mL |
| 72-95 lbs | 11 years | 3 tsp or 15 mL |
Other information
- each tsp contains: sodium 2 mg
- store at 15º to 30ºC (59º to 86ºF)
You may report serious side effects to: 130 Vintage Drive, Huntsville, AL 35811.
Inactive ingredients
D&C red #33, FD&C blue #1, flavor, glycerin, polyethylene glycol, propylene glycol, purified water, sodium benzoate, sorbitol solution, sucrose
Made in the USA
for Qualitest Pharmaceuticals
Huntsville, AL 35811
Rev. 7/09 R7
8073416 0840
PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL
| CHILDRENS QPAP
acetaminophen solution |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH NOT FINAL | part343 | 06/01/1997 | |
| Labeler - Qualitest Pharmaceuticals (011103059) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Vintage Pharmaceuticals-Huntsville | 958430845 | MANUFACTURE | |