DIBROMM WITH PE
-
brompheniramine maleate and
phenylephrine hydrochloride liquid
Major Pharmaceuticals
----------
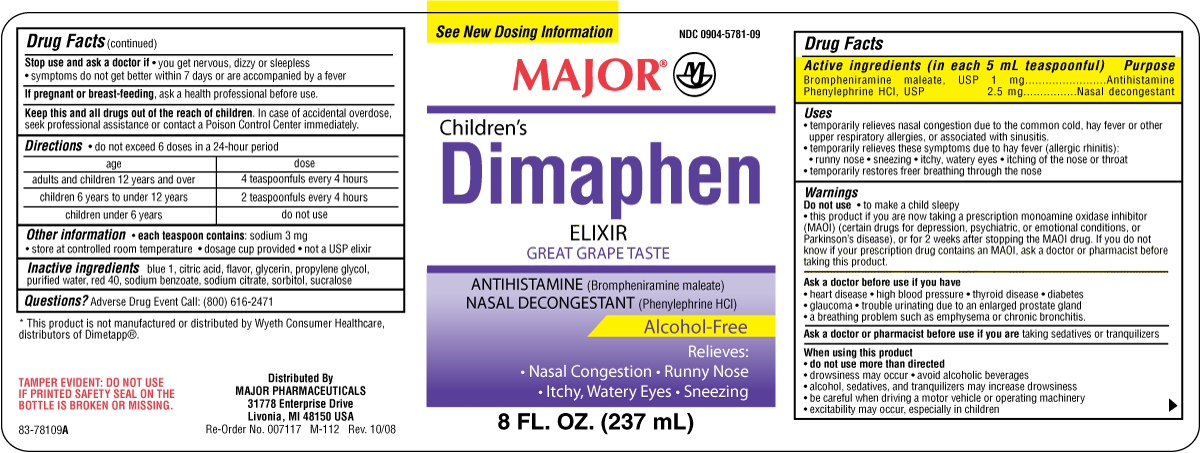
Drug FactsActive Ingredients
Brompheniramine maleate USP 1 mg
Phenylephrine HCl USP 2.5 mg
Purposes
Brompheniramine maleate....................Antihistamine
Phenylephrine HCl................................Nasal decongestant
Uses
• temporarily relieves nasal congestion due to the
common cold, hay fever or other upper
respiratory allergies, or associated with sinusitis.
• temporarily relieves these symptoms due to hay
fever (allergic rhinitis): • runny nose • sneezing
• itchy, watery eyes • itching of the nose or throat
• temporarily restores freer breathing
through the nose
Ask a doctor before use if you have
• heart disease • high blood pressure
• thyroid disease • diabetes • glaucoma
• trouble urinating due to an enlarged prostate gland
• a breathing problem such as emphysema or
chronic bronchitis
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers
When using this product
• do not use more than directed
• drowsiness may occur • avoid alcoholic beverages
• alcohol, sedatives, and tranquilizers may increase
drowsiness • be careful when driving a motor
vehicle or operating machinery • excitability may
occur, especially in children
Stop use and ask a doctor if
• you get nervous, dizzy or sleepless
• symptoms do not get better within 7 days
or are accompanied by fever
If pregnant or breast-feeding
ask a health professional before use.
Keep this and all drugs out of the reach of children.
In case of accidental overdose, seek professional
assistance or contact a Poison Control Center
immediately.
Directions
do not exceed 6 doses in a 24-hour period
age dose
adults and children 12 years and over........4 teaspoonfuls every 4 hours
children 6 years to under 12 years...............2 teaspoonfuls every 4 hours
children under 6 years...................................do not use
Questions?
Adverse Drug Event Call: (800) 616-2471
Other Information
• dosage cup provided
• each teaspoon contains: sodium 3 mg
• store at controlled room temperature
• not a USP elixir
Inactive Ingredients
blue 1, citric acid,
flavor, glycerin, propylene glycol, purified water,
red 40, sodium benzoate, sodium citrate, sorbitol,
sucralose
Principal Display Panel
Major
children's Dimaphen
Elixir
Great Grape Taste
Alcohol Free
8 FL OZ (237 mL)
| DIBROMM
WITH PE
brompheniramine maleate, phenylephrine hydrochloride liquid |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 01/07/2009 | |
| Labeler - Major Pharmaceuticals (191427277) |
| Registrant - Aaron Industries, Inc. (101896231) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Aaron Industries, Inc. | 101896231 | manufacture, analysis | |