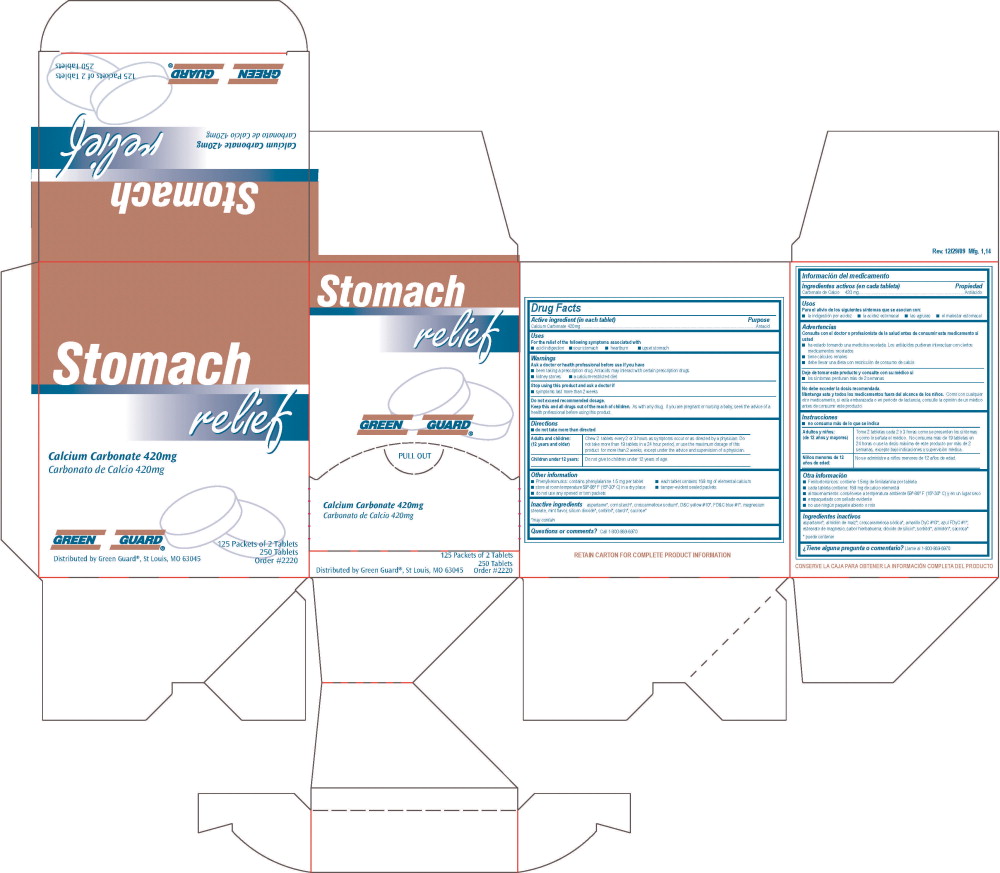
GREEN GUARD STOMACH RELIEF
-
calcium carbonate tablet, chewable
Unifirst First Aid Corporation
----------
Stomach Relief Green Guard®Drug Facts
Active ingredient (in each tablet)
Calcium Carbonate 420 mg
Purpose
Antacid
Uses
For the relief of the following symptoms associated with
- acid indigestion
- sour stomach
- heartburn
- upset stomach
Warnings
Ask a doctor or health professional before use if you have
- been taking a prescription drug. Antacids may interact with certain prescription drugs.
- kidney stones
- a calcium-restricted diet
Stop using this product and ask a doctor if
- symptoms last more than 2 weeks
Do not exceed recommended dosage.
Keep this and all drugs out of the reach of children. As with any drug, if you are pregnant or nursing a baby, seek the advice of a health professional before using this product.
Directions
- do not use more than directed
Adults and children: (12 years and older)
Chew 2 tablets every 2 or 3 hours as symptoms occur or as directed by a physician. Do not take more than 19 tablets in a 24 hour period, or use the maximum dosage of this product for more than 2 weeks, except under the advice and supervision of a physician.
Children under 12 years:
Do not give to children under 12 years of age.
Other information
- Phenylketonurics: contains phenylalanine 1.5 mg per tablet
- each tablet contains 168 mg of elemental calcium
- store at room temperature 59º-86º F (15º-30º C) in a dry place
- tamper-evident sealed packets
- do not use any opened or torn packets
Inactive ingredients
aspartame*, corn starch*, croscarmellose sodium*, D&C yellow #10*, FD&C blue #1*, magnesium stearate, mint flavor, silicon dioxide*, sorbitol*, starch*, sucrose*
*may contain
Questions or comments
Call 1-800-869-6970
Principal Display Panel - 101R Green Guard Stomach Relief Label
Stomach relief
Green Guard®
Pull Out
Calcium Carbonate 420 mg
Carbonato de Calcio 420mg
125 Packets of 2 Tablets
250 Tablets
Order #2220
Distributed by Green Guard®, St Louis, MO 63045
| GREEN GUARD STOMACH RELIEF
calcium carbonate tablet, chewable |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part331 | 12/30/2008 | |
| Labeler - Unifirst First Aid Corporation (832947092) |