MEDIQUE ASPIRIN
-
aspirin tablet, film coated
MEDI-FIRST ASPIRIN
-
aspirin tablet, film coated
MEDI FIRST PLUS ASPIRIN
-
aspirin tablet, film coated
Unifirst First Aid Corporation
----------
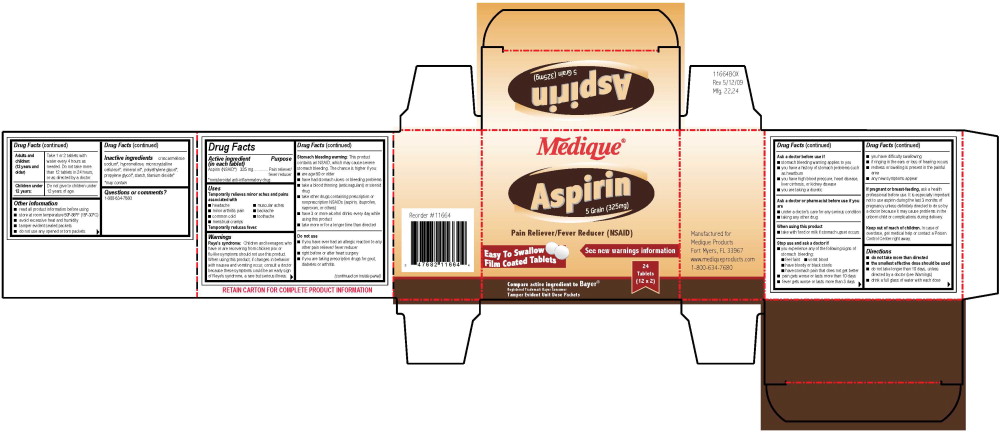
Aspirin (NSAID*) 325 mgDrug Facts
Active ingredient (in each tablet)
Aspirin (NSAID*) 325 mg
*nonsteroidal anti-inflammatory drug
Purpose
Pain reliever/fever reducer
Uses
Temporarily relieves minor aches and pains associated with
- headache
- minor arthritis
- common cold
- menstrual cramps
- muscular aches
- backache
- toothache
Temporarily reduces fever.
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription
NSAIDs (aspirin, ibuprofen, naproxen, or others) - have 3 or more alcohol drinks every day while
using this product - take more or for a longer time than directed
Do not use
- if you have ever had an allergic reaction to any other pain reliever/ fever reducer
- right before or after heart surgery
- if you are taking prescription drugs for gout, diabetes or arthritis
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
- under a doctor's care for any serious condition
- taking any other drug
When using this product
- take with food or milk if stomach upset occurs
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- you have difficulty swallowing
- if ringing in the ears or loss of hearing occurs redness or swelling is present in the painful area
- any new symptoms appear
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- do not take more than directed
- the smallest effective dose should be used
- do not take longer than 10 days, unless
directed by a doctor (see Warnings) - drink a full glass of water with each dose
Adults and children: (12 years and older)
Take 1 or 2 tablets with water every 4 hours as needed. Do not take more than 12 tablets in 24 hours, or as directed by a doctor.
Children under 12 years:
Do not give to children under 12 years of age.
Other information
- read all product information before using
- store at room temperature 59º-86ºF (15º-30ºC)
- avoid excessive heat and humidity
- tamper evident sealed packets
- do not use any opened or torn packets
Inactive ingredients
croscarmellose sodium*, hypromellose, microcrystalline cellulose*, mineral oil*, polyethylene glycol*, propylene glycol*, starch, titanium dioxide*
*may contain
Questions or comments?
1-800-634-7680
Principal Display Panel
116R Medique APAP 325 mg Label
Medique®
Aspirin
5 Grain (325 mg)
Pain Reliever/Fever Reducer (NSAID)
See new warnings information
East To Swallow
Film Coated Tablets
24 Tablets
(12 x 2)
Compare active ingredients to Bayer®
Registered Trademark Bayer Consumer
Tamper Evident Unit Dose Packets
116R MF Aspirin 325 mg Label
500 Tablets
(250 x 2)
Medi-First®
Aspirin
5 Grain (325 mg)
Aspirina 5 Granos (325 mg)
Pain Reliever/ Fever Reducer (NSAID)
Alivia el Dolor/ Reduce la Fiebre (AINE)
Easy To Swallow
Film Coated Tablets
Facil de Tragar Tabletas con Cubierta Pelicular
Pull to Open
Tire Para Abrir
See new warnings information
Compare active ingredients to:
Compare el ingrediente activo con:
Genuine Bayer®
Registered Trademark of Bayer Consumer
Marca Registrada de Bayer Consumer
Tamper Evident Unit Dose Packets
Empaquetado con Sellado
Evidente en Dosis Unitarias
116R MFP Aspirin 325 mg Label
100 Tablets
(50 x 2's)
Medi First® Plus
Aspirin
5 Grain (325 mg.)
Pain Reliever/Fever Reducer (NSAID)
Pull To Open
Easy To Swallow
Film Coated Tablets
See new warnings information
Compare Active ingredients to:
Genuine Bayer®
Registered Trademark of Bayer Corp.
Tamper Evident Unit Dose Packets
| MEDIQUE ASPIRIN
aspirin tablet, film coated |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part343 | 12/30/2008 | |
| MEDI-FIRST ASPIRIN
aspirin tablet, film coated |
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part343 | 12/30/2008 | |
| MEDI FIRST PLUS ASPIRIN
aspirin tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part343 | 12/30/2008 | |
| Labeler - Unifirst First Aid Corporation (832947092) |