CHILDRENS MUCUS RELIEF
-
guaifenesin liquid
H E B
----------
HEB Children's Mucus Relief Drug FactsActive ingredient (in each 5 mL tsp)
Guaifenesin, USP 100 mg
Purpose
Expectorant
Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Ask a doctor before use if the child has
- cough that occurs with too much phlegm (mucus)
- persistent or chronic cough such as occurs with asthma
Stop use and ask a doctor if
cough lasts more than 7 days, comes back, or occurs with fever, rash or persistent headache. These could be signs of a serious condition.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- do not take more than 6 doses in any 24-hour period
| Age | Dose |
| children 6 years to under 12 years | 1-2 teaspoonfuls every 4 hours |
| children 4 years to under 6 years | ½ - 1 teaspoonful every 4 hours |
| children under 4 years | do not use |
Other information
- each teaspoon contains: sodium 3 mg
- store at 20°-25°C (68°-77°F)
Inactive ingredients
acesulfame potassium, citric acid, FD&C blue no. 1, FD&C red no. 40, flavor, glycerin, maltitol, propylene glycol, purified water, saccharin sodium, sodium benzoate, sorbitol solution, sucralose, xanthan gum
Questions or comments?
1-800-719-9260
Principal Display Panel
Compare to Mucinex® for Kids active ingredient
For Ages 4 to 12
See New Dosing Directions
Children’s
Mucus Relief
expectorant – guaifenesin
Relieves Chest Congestion
Thins and Loosens Mucus
Grape Flavor Liquid
Alcohol Free
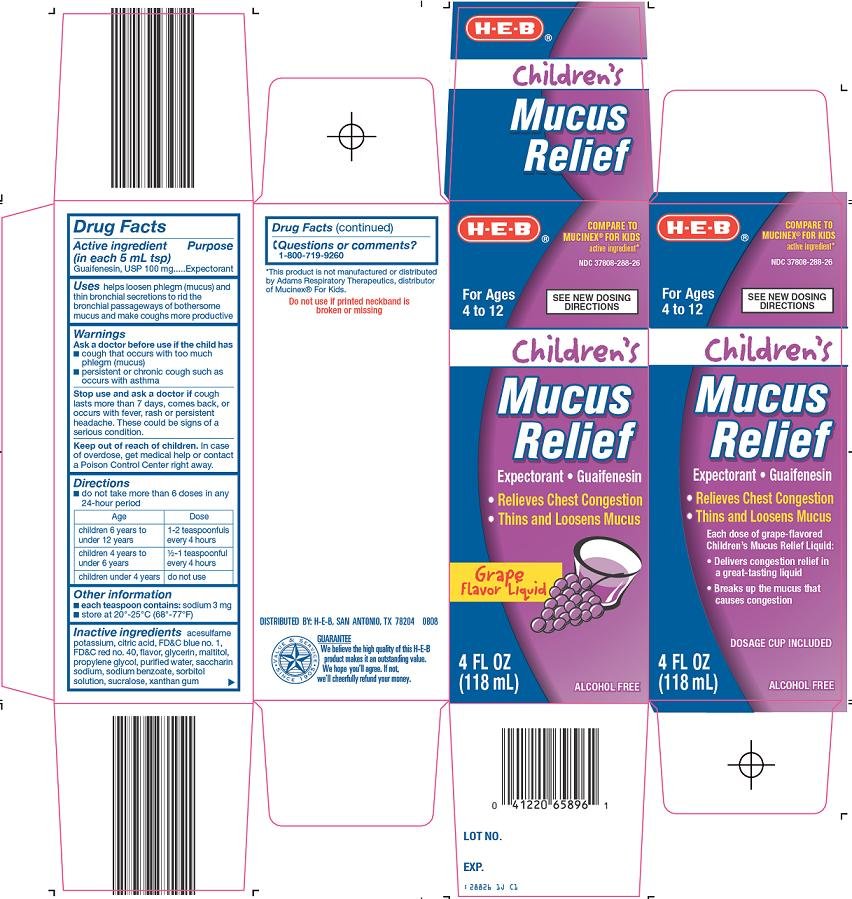
Children's Mucus Relief Carton
| CHILDRENS MUCUS RELIEF
guaifenesin liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 01/05/2009 | |
| Labeler - H E B (007924756) |