tigan (trimethobenzamide hydrochloride) suppository
tigan (trimethobenzamide hydrochloride) capsule
tigan (trimethobenzamide hydrochloride) injection
[Monarch Pharmaceuticals, Inc.]
Description
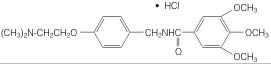
Chemically, trimethobenzamide HCl is N-[p-[2-(dimethylamino)ethoxy]benzyl]-3,4,5-trimethoxybenzamide monohydrochloride. It has a molecular weight of 424.93 and the following structural formula:
Capsules: Each 300-mg Tigan ® capsule for oral use contains trimethobenzamide hydrochloride equivalent to 300 mg. The capsule has an opaque purple cap marked“Tigan” and an opaque purple body marked “M079”.
Inactive Ingredients: D&C Red No. 28, FD&C Blue No.1, lactose, magnesium stearate, starch and titanium dioxide.
Suppositories (200 mg): Each suppository contains 200 mg trimethobenzamide hydrochloride and 2% benzocaine in a base compounded with polysorbate 80, white beeswax and propylene glycol monostearate.
Suppositories, Pediatric (100 mg): Each suppository contains 100 mg trimethobenzamide hydrochloride and 2% benzocaine in a base compounded with polysorbate 80, white beeswax and propylene glycol monostearate.
Ampuls: Each 2-mL ampul contains 200 mg trimethobenzamide hydrochloride compounded with 0.2% parabens (methyl and propyl) as preservatives, 1 mg sodium citrate and 0.4 mg citric acid as buffers and pH adjusted to approximately 5.0 with sodium hydroxide.
Multi-Dose Vials: Each mL contains 100 mg trimethobenzamide hydrochloride compounded with 0.45% phenol as preservative, 0.5 mg sodium citrate and 0.2 mg citric acid as buffers and pH adjusted to approximately 5.0 with sodium hydroxide.
Clinical Pharmacology
Mechanism of Action
The mechanism of action of Tigan ® as determined in animals is obscure, but may involve the chemoreceptor trigger zone (CTZ), an area in the medulla oblongata through which emetic impulses are conveyed to the vomiting center; direct impulses to the vomiting center apparently are not similarly inhibited. In dogs pretreated with trimethobenzamide HCl, the emetic response to apomorphine is inhibited, while little or no protection is afforded against emesis induced by intragastric copper sulfate.
Pharmacokinetics
The pharmacokinetics of trimethobenzamide have been studied in healthy adult subjects. Following administration of 200 mg (100 mg/mL) Tigan I.M. injection, the time to reach maximum plasma concentration (Tmax) was about half an hour, about 15 minutes longer for Tigan 300 mg oral capsule than an I.M. injection. A single dose of Tigan 300 mg oral capsule provided a plasma concentration profile of trimethobenzamide similar to Tigan 200 mg I.M. The relative bioavailability of the capsule formulation compared to the solution is 100%. The mean elimination half-life of trimethobenzamide is 7 to 9 hours.
Special Populations
Gender
Systemic exposure to trimethobenzamide was similar between men (N=40) and women (N=28).
Race
Pharmacokinetics appeared to be similar for Caucasians (N=53) and African Americans (N=12).
Indications and Usage
Tigan® is indicated for the treatment of postoperative nausea and vomiting and for nausea associated with gastroenteritis.
Contraindications
Use of the injectable form of Tigan ® in children, the suppositories in premature or newborn infants, and use of any dosage form in patients with known hypersensitivity to trimethobenzamide are contraindicated. Since the suppositories contain benzocaine they should not be used in patients known to be sensitive to this or similar local anesthetics.
Warnings
Caution should be exercised when administering Tigan® to children for the treatment of vomiting. Antiemetics are not recommended for treatment of uncomplicated vomiting in children and their use should be limited to prolonged vomiting of known etiology. There are two principal reasons for caution:
-
The extrapyramidal symptoms which can occur secondary to Tigan® may be confused with the central nervous system signs of an undiagnosed primary disease responsible for the vomiting, e.g., Reye’s syndrome or other encephalopathy.
-
It has been suspected that drugs with hepatotoxic potential, such as Tigan®, may unfavorably alter the course of Reye’s syndrome. Such drugs should therefore be avoided in children whose signs and symptoms (vomiting) could represent Reye’s syndrome.
Tigan® may produce drowsiness. Patients should not operate motor vehicles or other dangerous machinery until their individual responses have been determined.
Usage in Pregnancy:
Trimethobenzamide hydrochloride was studied in reproduction experiments in rats and rabbits and no teratogenicity was suggested. The only effects observed were an increased percentage of embryonic resorptions or stillborn pups in rats administered 20 mg and 100 mg/kg and increased resorptions in rabbits receiving 100 mg/kg. In each study these adverse effects were attributed to one or two dams. The relevance to humans is not known. Since there is no adequate experience in pregnant or lactating women who have received this drug, safety in pregnancy or in nursing mothers has not been established.
Usage with Alcohol:
Concomitant use of alcohol with Tigan ® may result in an adverse drug interaction.
Precautions
During the course of acute febrile illness, encephalitides, gastroenteritis, dehydration and electrolyte imbalance, especially in children and the elderly or debilitated, CNS reactions such as opisthotonos, convulsions, coma and extrapyramidal symptoms have been reported with and without use of Tigan® (trimethobenzamide hydrochloride) or other antiemetic agents. In such disorders caution should be exercised in administering Tigan®, particularly to patients who have recently received other CNS-acting agents (phenothiazines, barbiturates, belladonna derivatives). Primary emphasis should be directed toward the restoration of body fluids and electrolyte balance, the relief of fever and relief of the causative disease process. Overhydration should be avoided since it may result in cerebral edema.
The antiemetic effects of Tigan® may render diagnosis more difficult in such conditions as appendicitis and obscure signs of toxicity due to overdosage of other drugs.
Adverse Reactions
There have been reports of hypersensitivity reactions and Parkinson-like symptoms. There have been instances of hypotension reported following parenteral administration to surgical patients. There have been reports of blood dyscrasias, blurring of vision, coma, convulsions, depression of mood, diarrhea, disorientation, dizziness, drowsiness, headache, jaundice, muscle cramps and opisthotonos. If these occur, the administration of the drug should be discontinued. Allergic-type skin reactions have been observed; therefore, the drug should be discontinued at the first sign of sensitization. While these symptoms will usually disappear spontaneously, symptomatic treatment may be indicated in some cases.
Dosage and Administration
(See WARNINGS and PRECAUTIONS.)
Dosage should be adjusted according to the indication for therapy, severity of symptoms and the response of the patient.
CAPSULES , 300 mg
Usual Adult Dosage
One 300 mg capsule t.i.d. or q.i.d.
SUPPOSITORIES, 200 mg (not to be used in premature or newborn infants)
Usual Adult Dosage
One suppository (200 mg) t.i.d. or q.i.d.
Usual Children's Dosage
Under 30 lbs: One-half suppository (100 mg) t.i.d. or q.i.d.
30 to 90 lbs: One-half to one suppository (100 to 200 mg) t.i.d. or q.i.d.
SUPPOSITORIES, PEDIATRIC, 100 mg (not to be used in premature or newborn infants)
Usual Children's Dosage
Under 30 lbs: One suppository (100 mg) t.i.d. or q.i.d.
30 to 90 lbs: One to two suppositories (100 to 200 mg) t.i.d. or q.i.d.
INJECTABLE, 100 mg/mL (not for use in children)
Usual Adult Dosage
2 mL (200 mg) t.i.d. or q.i.d. intramuscularly.
NOTE: The injectable form is intended for intramuscular administration only; it is not recommended for intravenous use.
Intramuscular administration may cause pain, stinging, burning, redness and swelling at the site of injection. Such effects may be minimized by deep injection into the upper outer quadrant of the gluteal region, and by avoiding the escape of solution along the route.
Rx Only
Storage
Store at 25°C (77°F).
Excursions permitted to 15–30°C (59–86°F).
[See USP Controlled Room Temperature]
How Supplied
Capsules, 300 mg trimethobenzamide hydrochloride each, bottles of 100 and 500
NDC 61570-079-01 300 mg 100’s
NDC 61570-079-05 300 mg 500’s
Suppositories, Pediatric, 100 mg, boxes of 10
Suppositories, 200 mg, boxes of 10 and 50
NDC 61570-503-10 100 mg (box of 10)
NDC 61570-504-10 200 mg (box of 10)
NDC 61570-504-50 200 mg (box of 50)
Ampuls, 2 mL, boxes of 10
NDC 61570-540-02 100 mg/mL in 2 mL Ampul
Multi-Dose Vials, 20 mL
NDC 61570-541-20 100 mg/mL in 20 mL Multi-Dose Vials
Distributed By: Monarch Pharmaceuticals, Inc., Bristol, TN 37620
Manufactured By: King Pharmaceuticals, Inc., Bristol, TN 37620
Rev. 12/01
| Tigan (trimethobenzamide hydrochloride) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Tigan (trimethobenzamide hydrochloride) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Tigan (trimethobenzamide hydrochloride) | ||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Tigan (trimethobenzamide hydrochloride) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Tigan (trimethobenzamide hydrochloride) | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
Revised: 10/2006